



Even as the combined exports of the Indian pharma sector rose by 37 percent between January and November this year, Indian companies made international headlines for the wrong reasons after some countries reported adverse drug reactions and deaths of children who consumed cough syrups. After a long pause in US Food and Drug Administration (USFDA) inspections on account of the pandemic, inspections were back in full swing in 2023.
India's pharmaceutical exports saw a 37 percent (in terms of export value) rise from January to November, marking a positive trend. In terms of volume, the quantity was up 26 percent to over 63,000 tonnes in October 2023. India remained the largest generic drugs supplier to the US, contributing to almost 40 percent of the US market.
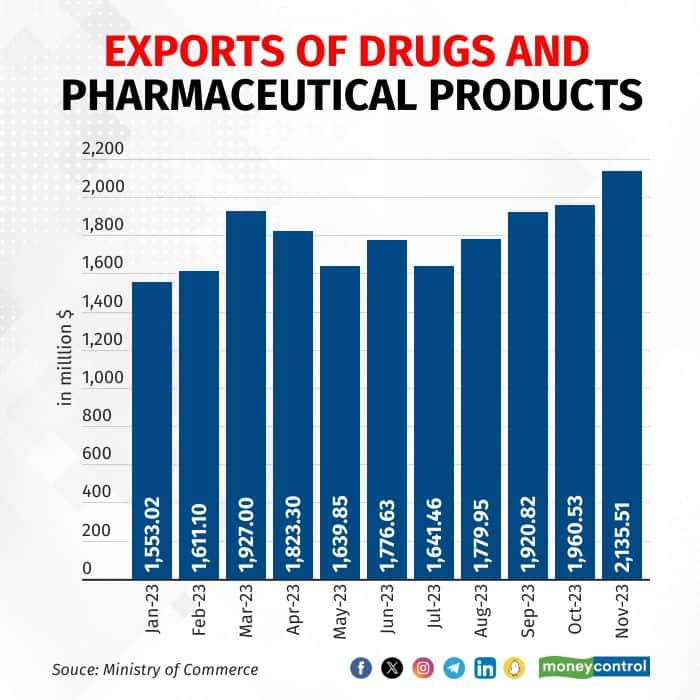
Many top Indian companies reported record high sales in the US this year. For Q2 FY 24 results, Cipla achieved record-breaking quarterly sales of $229 million. In the same period, Lupin’s US sales crossed $200 million for the first time in nearly the last two years, while Sun Pharma reported US formulation sales of $430 million.
Issues of substandard drugs continue
In late 2022, the World Health Organisation (WHO) issued an alert over child fatalities and adverse reactions to cough syrups in multiple countries, tracing some back to products originating in India. Throughout 2023, such incidents persisted, involving QP Pharma Chem's tainted syrups in the Marshall Islands and Micronesia, SyneCare in Nigeria, and the WHO flagging Fourrts Labs' cold Out in Iraq. Indiana Ophthalmics and Global Pharma faced serious allegations, including instances of eye damage in Sri Lanka and contamination in the US, linked to vision loss and a fatality.
The common factor in the cases involving cough syrups was the presence of dangerous levels of toxic substances, particularly diethylene glycol and ethylene glycol. Manufacturers often denied responsibility, claiming these ingredients were not used, or attributing the issues to improper product administration. In the case of the eye drops, the issues were caused by contamination.
Also read: Drug regulator issues warning against cold medication for children under 4 yrs
In response, Indian regulators took significant actions, inspecting 76 companies and revoking licenses of 18 of them producing spurious drugs. The government mandated rigorous testing of cough syrups before export, with the Central Drugs Standard Control Organization (CDSCO) temporarily pausing testing for other products. Regulators and industry associations, including the Indian Pharmaceutical Alliance (IPA) and the Indian Drug Manufacturers Association (IDMA), initiated training and education for Indian entities.
US FDA inspections surge after the Covid slowdown
After a long pause in US FDA inspection during the pandemic, inspections were back in full swing in 2023. Among the 189 inspections, 20 plants received the highest level of action, Official Action Indicated (OAI), up from 12 in 2022 and 5 in 2021.
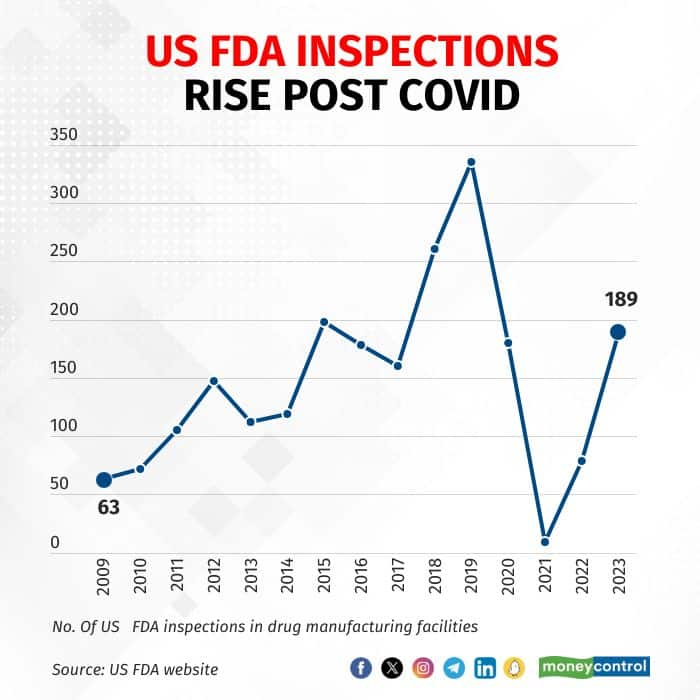 Number of US FDA inspections in Indian manufacturing facilities have increased post Covid
Number of US FDA inspections in Indian manufacturing facilities have increased post Covid
As Indian companies are focusing more on specialty drugs, the frequency of inspections is expected to increase. “Inspections are a necessary part of the lifecycle of these companies. As time passes the standards required by the regulator will also increase. It is natural,” noted Manoj Garg, Director of Investments, WhiteOak Capital.
US FDA Commissioner Robert M Califf had said in an interaction with CNBC TV 18 during his India visit in September, that the regulator will conduct more unannounced inspections.
A US Congressional committee had said that US FDA inspections in China and India are “inadequate”. It said that plants in the countries show a “pattern of repeatedly violating FDA safety regulations” and indulged in destroying or falsifying data, and using non-sterile manufacturing processes.
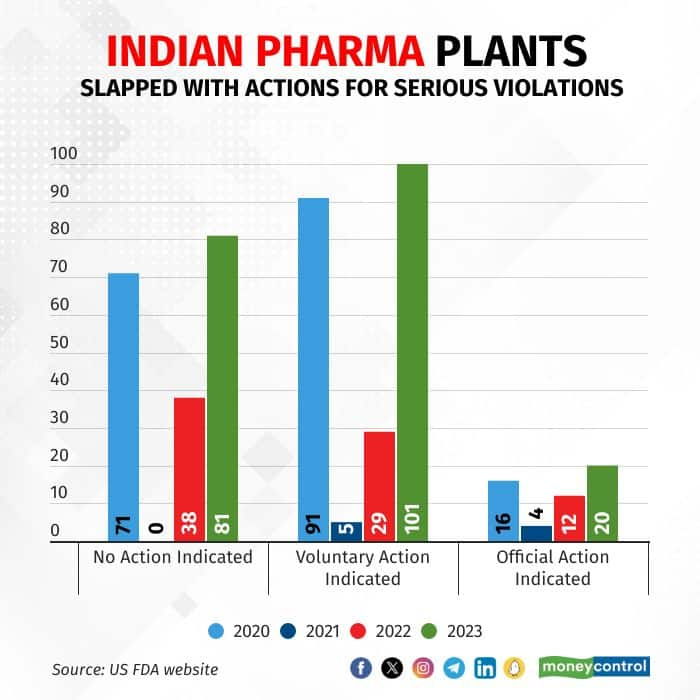 Number of serious regulatory actions against Indian pharma plants have increased in 2023
Number of serious regulatory actions against Indian pharma plants have increased in 2023
“If you look at pharma it is at that inflection point. We can draw a lot of parallels between the Indian pharmaceutical industry today and the IT industry in 2000,” said Gauri Chaudhari, author of `The Perfect Pill: 10 Steps to Build a Strong Healthcare Brand’. “There must be constant training and education on quality and strict inspections for the implementation of the quality standards to help pharma take a leap like what IT took in the early 2000s,” she added.
Also read: US FDA to step up unannounced inspections on Indian pharma plants
Discover the latest Business News, Sensex, and Nifty updates. Obtain Personal Finance insights, tax queries, and expert opinions on Moneycontrol or download the Moneycontrol App to stay updated!
Find the best of Al News in one place, specially curated for you every weekend.
Stay on top of the latest tech trends and biggest startup news.