



Dr Reddy’s Laboratories share price gained intraday on December 2 after the company commenced adaptive phase 2/3 clinical trials for Sputnik V vaccine in India after receiving the necessary clearance from the Central Drugs Laboratory, Kasauli.
This will be a multicenter and randomized controlled study, which will include safety and immunogenicity study, said the company.
The clinical trials are being conducted by JSS Medical Research who are the clinical research partner.
Further, Dr Reddy’s has partnered with the Biotechnology Industry Research Assistance Council (BIRAC), Department of Biotechnology (DBT) for advisory support and to use BIRAC’s clinical trial centres for the vaccine.
In September 2020, Dr Reddy’s and RDIF entered into a partnership to conduct clinical trials of the Sputnik V vaccine and the rights for distribution of the first 100 million doses in India.
"This is another significant step as we continue to collaborate with multiple entities along with the government bodies to fast-track the process for launching the vaccine in India. We are working towards making the vaccine available with a combination of import and indigenous production model,” said G V Prasad, Co-chairman and Managing Director, Dr Reddy’s Laboratories.
At 09:55 hrs, Dr Reddys Laboratories was quoting at Rs 4,840, up Rs 9.60, or 0.20 percent on the BSE.
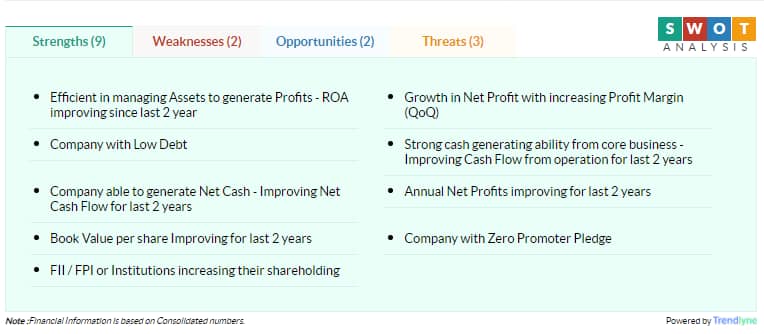
The share touched its 52-week high Rs 5,514.65 and 52-week low Rs 2,497.60 on 21 September 2020 and 19 March 2020, respectively.
Currently, it is trading 12.23 percent below its 52-week high and 93.79 percent above its 52-week low.
Discover the latest Business News, Sensex, and Nifty updates. Obtain Personal Finance insights, tax queries, and expert opinions on Moneycontrol or download the Moneycontrol App to stay updated!
Find the best of Al News in one place, specially curated for you every weekend.
Stay on top of the latest tech trends and biggest startup news.