


The Director General of Foreign Trade (DGFT) announced on February 24 that it has made Remdesivir Injection and API, Amphotericin-B Injections, Enoxaparin (Formulation and API), and Intra-Venous Immunoglobulin (IVIG) as ‘freely’ exportable, effective immediately.
The announcement comes at a time the COVID-19 situation in the country is relatively under control with declining daily coronavirus cases and limited hospitalisations.
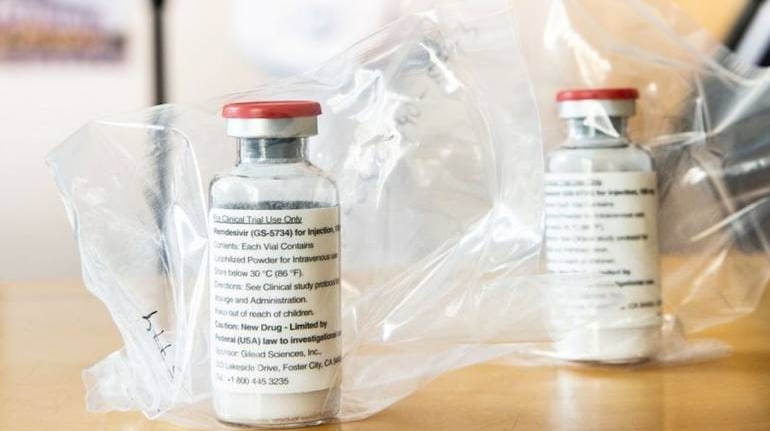
Until now, the Government of India used to allow restricted export of injection Remdesivir and Remdesivir Active Pharmaceutical Ingredients (API) – a drug that is used in COVID-19 treatment.
ICMR says Remdesivir 'not a life-saving drug', calls for safe and rational usage
In the wake of the second wave of the pandemic, when COVID-19 cases had increased sharply, the Centre had, on April 11, halted the export of Remdesivir injection and API. At the time, there was a growing demand for Remdesivir injections in the country.
Remdesivir is an injectable antiviral drug produced by Gilead Sciences Inc. It is the first drug to have received approval from the US Food and Drug Administration (FDA) in October 2020, making it the first medicine for treating COVID-19 infections. It is said to shorten the recovery of the patients by controlling the multiplication of the virus within.
Seven Indian companies - Mylan, Hetero, Jubiliant Life Sciences, Cipla, Dr Reddy's, Zydus Cadila and Sun Pharma – produce Remdesivir injections under licensing agreement with Gilead Sciences. The total installed capacity of the seven manufacturers of Remdesivir was 38.80 lakh vials per month until the government had stepped in last year and announced setting up of seven additional sites with a production capacity of 10 lakh vials per month to tackle the sharp rise in demand of the drug during the second wave of the pandemic.
Discover the latest Business News, Sensex, and Nifty updates. Obtain Personal Finance insights, tax queries, and expert opinions on Moneycontrol or download the Moneycontrol App to stay updated!
Find the best of Al News in one place, specially curated for you every weekend.
Stay on top of the latest tech trends and biggest startup news.