


The novel coronavirus continues to be on a rampage across the globe, having affected at least 4 million people and taken the lives of at least 280,000.
What makes this virus dangerous is its novelty and how little is known about it to scientists, who are carrying out extensive research to study the virus and how to kill it.
In the interim, various treatments are being tried out to help subside the symptoms of the COVID-19 infection; some have even yielded positive results. The World Health Organisation (WHO) has launched ‘Solidarity Clinical trial’ to help find an effective treatment of COVID-19. The trial compares treatment options against standard of care to rapidly discover whether any of the drugs slow disease progression or improve survival.
However, the medical body has cautioned against doctors recommending or administering unproven treatments to COVID-19 patients, as it has also cautioned patients against self-medicating.
To understand what all treatments are under trial and have shown positive outcomes, let’s recollect what the coronavirus does when it enters our body.
What does coronavirus do?
The novel coronavirus spreads through droplets of an infected person, or from contact of infected surfaces. After it enters the human body, the spiked proteins sticking out of its surface latch on to our cell membranes and hijack them – asking our cells to stop performing their usual function and use all resources to help the virus multiply.
Consequently, when it enters the lungs through the throat and the bronchial tubes, it disrupts the functioning of the alveoli – which absorb oxygen from air into the blood, and take carbon dioxide from the blood into the air – causing great difficulty in breathing.
Patients with acute symptoms of COVID-19, such as pneumonia, have to be put on ventilators.
Ventilators
A ventilator takes over the body’s breathing process in cases where lungs have failed due to an infection. This gives the patient time to fight off the infection and recover. Ventilators can be broadly classified into two types – mechanical and non-invasive.
In the mechanical type, a tube is inserted into the airway of the patient, to bring oxygen matched with the patient’s body temperature and moisture in air. Another tube in the machine takes the carbon dioxide from the air that the patient exhales.

In the non-invasive type, people with milder symptoms are given air with increased levels of oxygen using face masks, nasal masks, or mouthpieces.
Several other drugs/therapies are being clinically tested for the treatment of COVID-19, even though they weren’t really developed with coronavirus in mind. These repurposed drugs include:
Hydroxychloroquine (HCQ)
Hydroxychloroquine is a drug used in the treatment of malaria. Doctors also prescribe HCQ for the treatment of rheumatoid arthritis (inflammation of joints) and lupus (an inflammatory disease when the immune system attacks its own tissues).
HCQ came into the picture after US President Donald Trump touted it to be a “game changer”. His statement led to panic buying, and hence shortage of the drug for arthritis and lupus patients.
The Indian Council for Medical Research (ICMR) has, however, allowed doctors to prescribe it only in select cases, such as household contacts of people who have tested positive for coronavirus.
Favipiravir
Favipiravir is an antiviral drug, originally used for the treatment of influenza. It received regulatory approval in Japan in 2014 and was marketed as Avigan. It was used to treat symptoms during the Ebola epidemic of 2014-16 as well as during the MERS outbreak.
Since the novel coronavirus (SARS-CoV-2) is an RNA virus like the influenza A and B viruses, Favipiravir could also work against the former.
Favipiravir, which stops viral replication in infected cells, is being used for clinical trials in several countries, including China, which said the drug was “very safe and clearly effective” in treating 340 patients.
Tocilizumab
Tocilizumab is an immune-modulator, which is used in treating auto-immune diseases – diseases in which the immune system mistakenly starts attacking the body – such as rheumatoid arthritis and giant cell arteritis.
Tocilizumab is effective in preventing extreme inflammation in critically-ill patients.
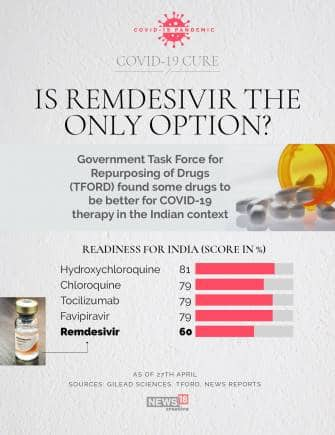
The Task Force of Repurposing of Drugs have given Favipiravir and Tocilizumab a Drug Potential score of 75 percent, as well as 79 percent drug readiness score for how readily they can be manufactured and distributed.
This is the highest for the 19 treatments analysed so far.
Sepsivac
Originally used to treat leprosy, Sepsivac is now being repurposed for the treatment of COVID-19 by Cadilla Pharmaceuticals.
Sepsivac was first used to treat leprosy in India in 1966. Now, it has helped treat for coronavirus patients in Chandigarh. Phase-III human trials of Sepsivac are underway, with over 4,500 people.
Remdesivir
Remdesivir is a broad spectrum antiviral that was used for the treatment of Marburg, MERS and SARS viruses, both in vitro and in animal models. It works by disabling virus RNA replication. RNA is the genetic material used by novel coronavirus to replicate itself.
Although it failed to treat Ebola, for which it was purposed, US drug major Gilead Sciences is now repurposing the drug to treat coronavirus. Initial trials have revealed that Remdesivir reduced the recovery time from 15 days to 11 days.
Interferon
Interferon was first developed in the 1980s by Cuba to treat dengue. Since then, it has been repurposed for kidney cancer, leukaemia and hepatitis A and B. It is an anti-viral drug which acts by boosting the immunity of the patient against the infection.
ICMR has approved the clinical trial of the drug in Kerala.
Plasma Therapy
When a person contracts the COVID-19 infection, their body produces antibodies to attack the virus. These antibodies are secreted by immune cells, called B lymphocytes, found in the plasma, the liquid part of the blood. If the infected person can produce sufficient antibodies, he can recover from the disease caused by the virus.
Once a person has recovered, the antibodies continue to stay in the blood, waiting to fight the virus should it return.
In plasma therapy, the plasma of a person, who has recovered from COVID-19, and thus has sufficient antibodies to fight the disease, is drawn and transferred to people who have freshly contracted the disease.
The procedure is also being used in very select cases and for clinical trials.
Antiviral Therapy
A two-week course of an antiviral therapy, if started within seven days of contracting coronavirus, can improve clinical recovery, a clinical trial in Hong Kong has shown.
The therapy uses a combination of combination of the drugs interferon beta-1b, lopinavir-ritonavir and ribavirin.
A combination of the drugs lopinavir-ritonavir, normally used to treat HIV, and ribavirin, an oral hepatitis-C virus drug, had significantly reduced respiratory failure and death in patients hospitalised with SARS during the 2002-03 outbreak.
In addition, Interferon beta-1b, which was developed to treat multiple sclerosis (MS), had been shown to reduce viral load and improve lung problems in animal studies of MERS coronavirus infection.
Out of the multitude of drugs available, Artificial Intelligence as well as machine learning are being used to identify drugs for repurposing. The science and technology ministry has reportedly earmarked 42 existing drugs, narrowed down from a list of at least 2,100 approved drugs, for repurposing.
Discover the latest Business News, Sensex, and Nifty updates. Obtain Personal Finance insights, tax queries, and expert opinions on Moneycontrol or download the Moneycontrol App to stay updated!
Find the best of Al News in one place, specially curated for you every weekend.
Stay on top of the latest tech trends and biggest startup news.