



A recent government decision to hike the monetary support offered to patients suffering from rare diseases from Rs 20 lakh to 50 lakh, covering all conditions, is set to pave the way for the introduction of new therapies and drugs for the treatment of ‘orphan diseases’ in the country. In a recent order, the Union health ministry said that financial support up to Rs 50 lakh would be provided to the patients from any category of rare diseases at the specified centres of excellence.
This is a departure from its previous line of supporting patients of only specified diseases with a one-time grant of up to Rs 20 lakh. Also, these patients, in order to be eligible for the grant, needed to be beneficiaries of the government’s flagship health insurance scheme, Ayushman Bharat Pradhan Mantri Jan Arogya Yojana.
The change in policy has come as a boon not just for patients but also pharma companies looking to introduce various rare drug therapies in India.
“I am hopeful that the move will substantially raise access to treatment for thousands and thousands of patients of rare diseases in India,” said Prasanna Shirol, who runs an umbrella body, Organisation for Rare Disease in India, which works for the rights of patients with rare diseases.
He pointed out that many companies do not bring their drugs meant for rare diseases in India due to their unaffordability.
Another activist, who has been working in the field for long, pointed out that patients of conditions such as Hurler syndrome (a disease in which the body cannot digest sugar), Pompe disease (in which muscles and nerves are damaged severely) or Hunter disease (when sugar molecules build up inside tissues) can get a shot at treatment. The activist did not want to be identified.
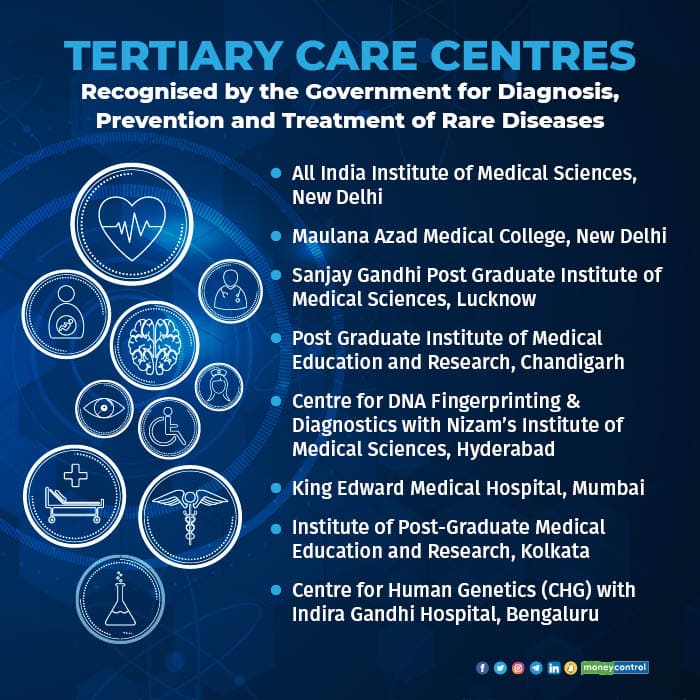
Rare diseases in India
Rare diseases are mostly life-threatening and chronic and the World Health Organisation defines a rare disease as a debilitating, lifelong condition with a prevalence of 1 or less per 1,000 populations.
A hospital-based registry of rare disease patients, being prepared by the Indian Council of Medical Research, is yet to come up but there are estimates that there may be at least 7-8 crore patients of rare diseases in India.

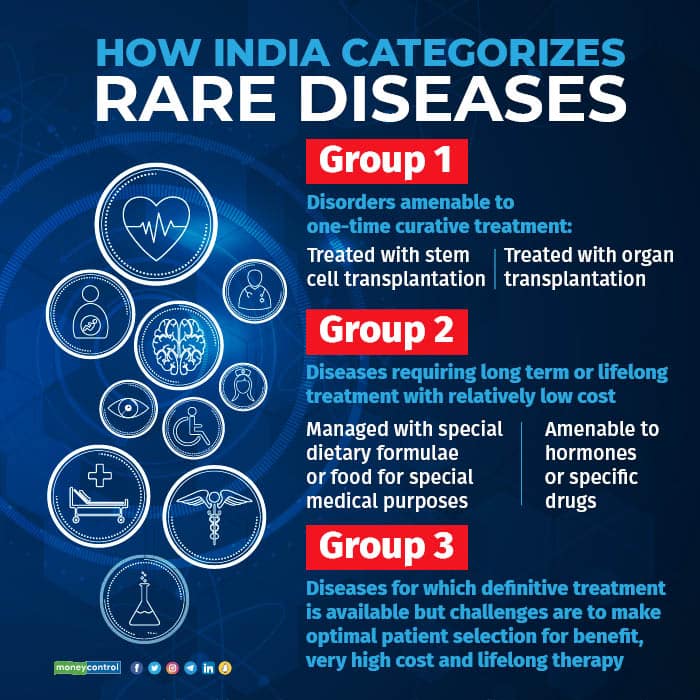
There are over 700 diseases categorised as rare diseases but a 2021 policy document by the Union health ministry said that less than 5 percent have therapies available to treat them.
Most of these diseases are genetic in nature and babies born with these conditions, if they survive, may need transplants or later lifelong support or treatment, depending on their condition.
About 95 percent of rare diseases have no approved treatment in India and less than 1 in 10 patients receive disease-specific treatment.
Prohibitive cost of treatment
The ministry had said last year that while in many cases drugs for most diseases were not available, and even where they are, they are “prohibitively expensive, placing immense strain on resources”.
Barely any drug for rare diseases is made in India and all such medicines have to be imported.
A paper published in a scientific journal on rare diseases had also said that a majority of patients with rare diseases in India cannot afford the high costs of treatments or drugs, often called orphan drugs, even when available.
“Most orphan drugs are not curative but palliative, and need to be administered regularly,” it added.
Shirol said that lifelong treatments and drugs of many rare diseases can run in crores of rupees.
“Some rare disease patients may need lifelong therapies which are either not available in India or are too costly,” he added.
Advanced treatment for many rare diseases likely in India now
Some pharma companies meanwhile said that they will try to quickly introduce advanced treatments for rare diseases in India.
“Globally, the company has developed a portfolio of several therapies to treat various rare diseases and our dedicated research unit is also working on developing therapies for various rare diseases with a strong focus on gene therapies,” said S Sridhar, country manager for Pfizer India.
“At Pfizer, we continuously evaluate our go-to-market model to bring in medicines and treatments specific to India, coupled with innovative access plans that offer pricing that suits the Indian market,” he said.
Also read: Why are young Indians increasingly dying of heart diseases?
This, according to Sridhar, is to ensure that the company makes these medicines available to as many patients as possible.
“Support from the government, through the current and future development of the rare disease policy will certainly help us further enhance this access,” he added.
K G Anathakrishnan, director general of the Organisation of Pharmaceutical Producers of India, a network of multinational pharma companies, too welcomed the move, saying such initiatives will help create a truly patient-centred ecosystem and ensure equal and quality healthcare for patients suffering from rare diseases.
“As an industry, we stand with the government in the fight against rare diseases and are fully committed to advanced treatments and make them available to patients as fast as possible. This policy will help enhance access to patients,” Anathakrishnan said.
The organisation’s members, said Anathkrishnan, are committed to address unmet medical needs through cutting-edge research and will continue to develop treatments for rare diseases, which they recognise as a public health challenge.
Sudarshan Jain, secretary general of the Indian Pharmaceutical Alliance, a network of research-intensive pharma companies in India, too said that the hike in financial aid has come as a major relief to the stressed families of rare disease patients in the country.
Discover the latest Business News, Sensex, and Nifty updates. Obtain Personal Finance insights, tax queries, and expert opinions on Moneycontrol or download the Moneycontrol App to stay updated!
Find the best of Al News in one place, specially curated for you every weekend.
Stay on top of the latest tech trends and biggest startup news.