



The Halol unit of India’s largest drugmaker Sun Pharma, which has not received new product approvals from US regulators ever since getting warning letters from them in 2015, is unlikely to see a resolution of the problem this calendar year, contrary to many analysts’ expectations.
Production at Halol, one of Sun’s largest manufacturing facilities, accounted for 40 percent of US sales, and the action by the US Food and Drug Administration has hit the company’s stock price over the last two years.
A Sun spokesman said in an emailed response to a Moneycontrol News questionnaire: “We are fully committed to bringing Halol back into compliance. For this, we have submitted our response and corrective action plan to the US FDA. We are now in the process of implementing the corrective steps. We are working towards addressing this over the next few quarters; however, we have not given any specific timeline as it is difficult to do so.”
The Halol unit, which manufactures injectibles and oral solids, was issued the letters following a surprise inspection carried out by the US FDA in September 2014. The company roped in consultants to address the concerns and after a lengthy remediation process invited the regulator for another round of inspections in November 2016. The second inspection also resulted in nine observations under Form 483.
Experts say that since the second inspection also resulted in some observations, the company will not invite a third inspection in a hurry. While none of the nine observations are repeat observations, a resolution would definitely take longer than six months. Globally, resolutions typically take at least 500 days and the data for India and China is worse. So far, only one warning letter has seen a resolution out of 64 issued to Indian and Chinese companies in the last 52 months. As the table below shows, resolution at Wockhardt is still pending, over 1,300 days since warning letters were issued.
Several analysts are factoring in a revival in Sun’s base business in FY18 as Halol would see resolution. For instance, IIFL Research says, “We believe Sun can resolve Halol issues in six months, which will lead to resumption of product approvals and will enable it to grow US revenues from a shrunken base.”
Sales of Sun Pharma’s FY16 US sales fell to USD 2.06 billion from USD 2.24 billion in FY15, as production at Halol dropped due to remedial action undertaken by the company at the production unit.
Table: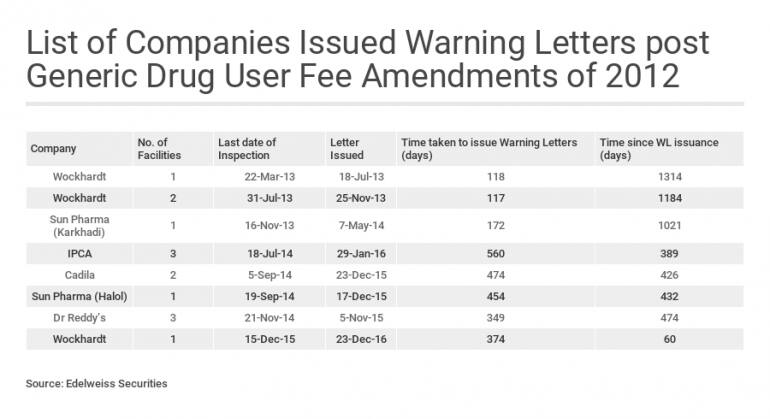
Discover the latest Business News, Sensex, and Nifty updates. Obtain Personal Finance insights, tax queries, and expert opinions on Moneycontrol or download the Moneycontrol App to stay updated!
Find the best of Al News in one place, specially curated for you every weekend.
Stay on top of the latest tech trends and biggest startup news.