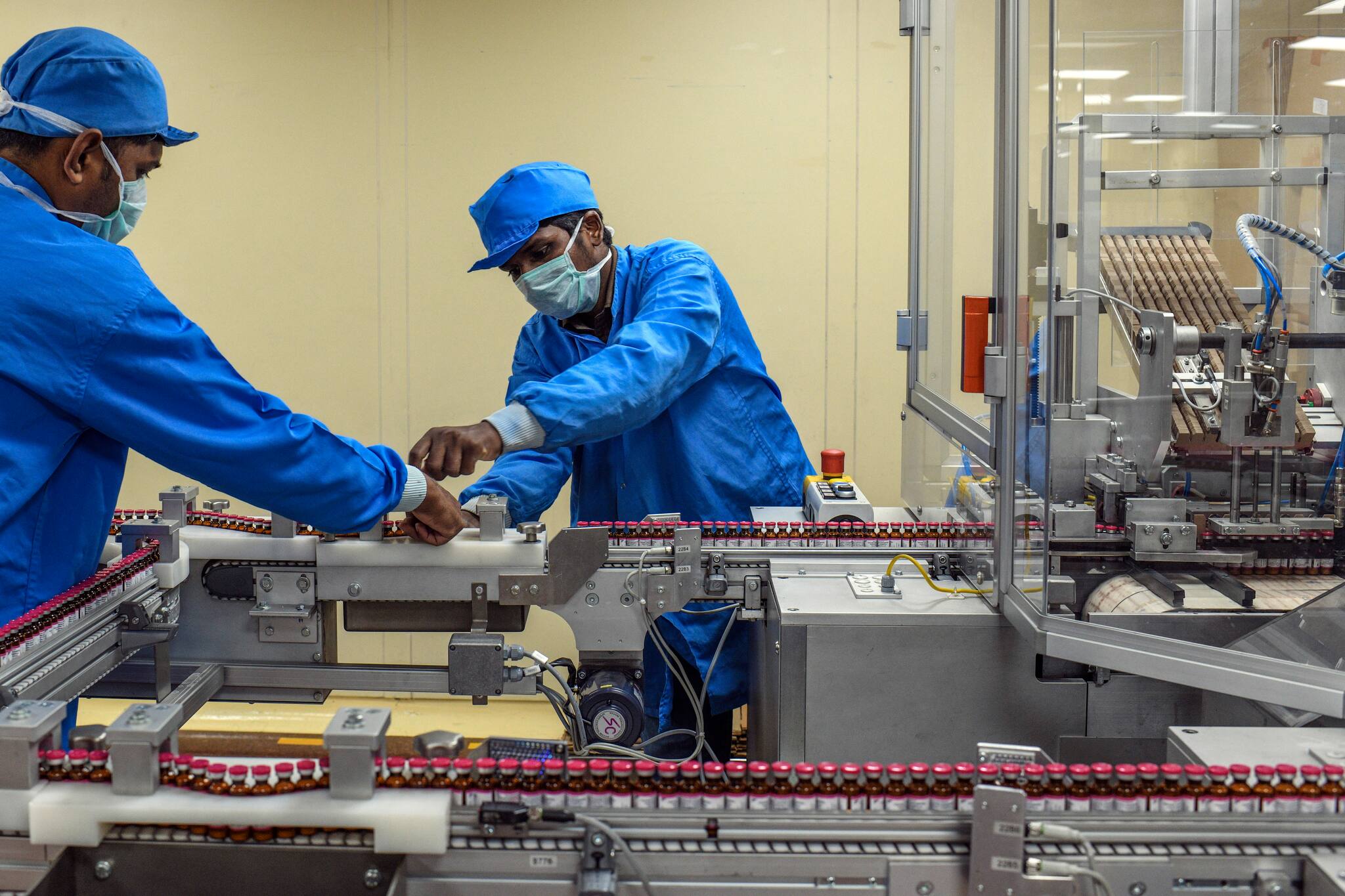
BUSINESS
Why fire at Serum Institute of India's Pune plant has sent jitters across vaccine industry
The companies that are making COVID-19 vaccines can be highly vulnerable targets for cyber attacks and other sophisticated attempts of sabotage, say experts.

BUSINESS
Bharat Biotech's Covaxin found to be safe, induced immune response in Phase-1
The overall incidence of 'adverse events' was 14–21 percent in all vaccine-treated groups, says the data published in Lancet. The company says the incidence of adverse events are noticeably lower than the rates for other SARS-CoV-2 vaccine platform candidates.

BUSINESS
Alembic Pharma head Amin says confident of sustaining growth momentum
Analysts tracking the company say Alembic’s growth in the US will depend on new product launches and commercialisation of its injectable portfolio.

BUSINESS
Status check on COVID-19 vaccines in pipeline for India over next six months
In the initial phase, India will inoculate about one crore healthcare workers. This will be followed by two crore frontline workers, police, armed forces, municipal workers, revenue staff and others. In the third phase, 27 crore people above 50 years of age and those with co-morbidities.
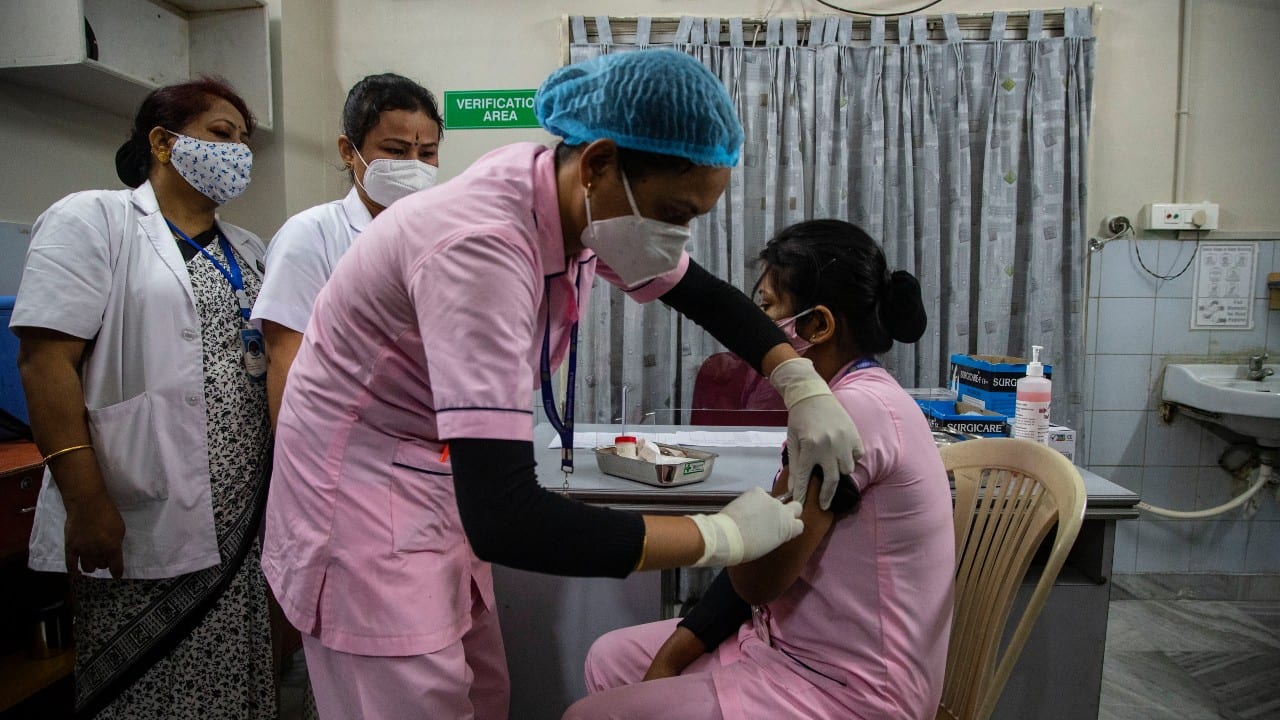
BUSINESS
Why potential vaccine-related legal liabilities worry Covid-19 vaccine makers
As part of their purchase pacts, some countries have agreed to indemnify vaccine makers against claims following any adverse effects. India has not agreed to this so far. Executives of vaccine makers are also worried about the high cost of product liability covers they will have to buy given the limited data on adverse effects at this stage
BUSINESS
62% Indians hesitant to get COVID-19 vaccine shot: LocalCircles survey
As per LocalCircles, the COVID-19 vaccine survey is based on 17,000 responses received from citizens residing in 230 districts of India.

BUSINESS
Around 3.81 lakh people vaccinated, govt says no cases of serious AEFI linked to vaccine found till date
So far, around 580 cases of AEFI have been reported, of which there were seven cases of hospitalisation.

BUSINESS
Why Johnson & Johnson's Covid-19 vaccine is generating a lot of interest
Initial reports indicate the vaccine elicits a very good response. The single-dose vaccine can prove to be a gamechanger, especially in a country with a large population, such as India, where two doses will consume tremendous resources

BUSINESS
Pharma wrap: India rolls out COVID-19 vaccination of healthcare workers, needs to address technical glitches, vaccine hesitancy
Moneycontrol spoke to couple of doctors who received Covid-19 vaccine shot. All of them expressed satisfaction on the way things went.

BUSINESS
What is informed consent form for Bharat Biotech Covaxin, what information is sought in the form and other questions answered
..people who receive the vaccine will have to sign a three-page informed consent form, and will also be monitored for any serious side effects.
BUSINESS
Explained | COVID-19 vaccination drive: How to register, who will get the shots first and other queries answered
In the initial phase, the government said it would inoculate about one crore healthcare workers. This will be followed by two crore frontline workers, police, armed forces, municipal workers, revenue staff and others.

BUSINESS
Here are 10 things to know about SII's Covishield vaccine from package insert & factsheet
The package insert and factsheet the comes with the vaccine includes information on indications and usage, dosage strength, clinical efficacy, contraindications, risks and benefits, and use in specific populations.
BUSINESS
Dr Reddy's gets DSMB nod to conduct Phase-3 trial of Sputnik Covid-19 vaccine in India
The phase 2 study of Sputnik V was conducted on 100 subjects as part of the randomised, double-blind, parallel-group, placebo-controlled study in India. The DSMB concluded that no safety concerns were identified and the study has met the primary endpoints of safety, Dr Reddy's said.

BUSINESS
Pharma wrap: Why Bharat Biotech's Covaxin trial site in Bhopal is stirring up controversy
There were allegations of certain violations in the Phase-III clinical trial taking place at People’s College of Medical Sciences and Research Centre in Bhopal to assess the efficacy and safety of the vaccine.

BUSINESS
PM's scientific advisor says current vaccines offer immunity against Covid-19 for at least a year
"The expectation is that the immunity due to the vaccines will be significantly long, certainly we think over a year, but how long we don't know," VijayRaghavan said addressing questions on Covid-19 vaccines.

BUSINESS
Government to give priority to suppliers sourcing maximum local input materials in procurement of medicines
A Class-I supplier will use local content equal to or more than 80 percent, Class-II supplier use 50-80 percent of local content and Non-Local supplier will use less than 50 percent. Class-I and Class-II suppliers will get higher priority.

BUSINESS
Bharat Biotech completes enrolment of 25,800 volunteers for phase-3 trial of Covaxin
Bharat Biotech expects the administration of the second dose of Covaxin by February-March, said Chairman and Managing Director Krishna Ella.

BUSINESS
Here are 10 things to know about Bharat Biotech, its founder Krishna Ella
Ella said Bharat Biotech is investing Rs 100 crore on research and conducting clinical trials in FY2021. In addition, the company will invest up to Rs 200 crore in FY2021 and FY2022 for the development of a greenfield facility for a Covid-19 vaccine.

BUSINESS
Here’s how SII’s Covishield and Bharat Biotech’s Covaxin stack up against each other
How safe are the vaccines, how many doses do you need to take, what is the duration between doses, what is the price of the vaccines, which countries have approved them… Read on to get the answers to these and other questions.

BUSINESS
Panel reviewed Bharat Biotech Covaxin in the absence of efficacy data, show minutes of SEC meeting
The minutes of the SEC meeting show that the panel had asked Bharat Biotech to expedite the recruitment for phase-3 trials and perform interim efficacy analysis, even as it recommended the restricted emergency use of Covaxin on January 1.

BUSINESS
DCGIs asks Bharat Biotech to submit protocol for administering COVID-19 vaccine under clinical trial mode
The clinical trial mode means, that everyone will consent for the vaccine, there will be no placebo arm and the recipients will be closely monitored, said ICMR's Balram Bhargava.

BUSINESS
Covaxin approval: Beyond the emotional outburst, here’s what Bharat Biotech’s Krishna Ella said about the vaccine
The approval of Covaxin, which is still undergoing phase-3 clinical trials and yet to have efficacy data, has been roundly criticised. But Krishna Ella, Chairman and Managing Director of Bharat Biotech, the maker of Covaxin, feels a lot of that criticism is not justified. Here’s a look at what he had to say about its efficacy and immunogenicity data, administering it in ‘clinical trial mode’ and whether it is just a ‘back-up’ vaccine

BUSINESS
Explained: Why conditional approval for Bharat Biotech’s Covaxin is raising questions
The DCGI has said the approval is in “clinical trial mode” but has not clarified this in detail. If an experimental vaccine is given to people, there should be informed consent explaining the potential risks and benefits of the vaccine and post-vaccination follow-up. In case there is any serious adverse reaction, the recipient may also be eligible for compensation.

BUSINESS
Coronavirus vaccine India | Covaxin offers protection against mutant COVID-19 strains, says Bharat Biotech, ICMR
Bharat Biotech said the evaluation of its coronavirus vaccine Covaxin has resulted in several unique product characteristics including long term persistence of immune responses to multiple viral proteins, as opposed to only the spike protein, and has demonstrated broad spectrum neutralizing capability with heterologous SARS-CoV2 strains, thus potentially reducing or eliminating escape mutants.










