
BUSINESS
Zydus Cadila seeks permission from DCGI to test its COVID-19 antibody cocktail on humans
Zydus said the monoclonal antibody cocktail ZRC-3308 has been found to be safe and well tolerated in animal toxicology studies and has demonstrated the ability to neutralise SARS-CoV-2 both in vitro and in animal studies.

BUSINESS
GSK says exploring all options to make COVID-19 antibody drug Sotrovimab available in India quickly
Treatment with Sotrovimab resulted in an 85 percent reduction in the risk of hospitalisation or death in high-risk adult COVID-19 outpatients compared to placebo, based on interim results from Phase 3 COMET-ICE trial.
BUSINESS
Samsung to supply one million LDS syringes to help cut COVID-19 vaccine wastage
The technology behind LDS syringes demonstrated up to 20 percent greater efficiency. If existing syringes were to deliver one million doses, LDS syringes could deliver 1.2 million doses with the same amount of vaccine, the company said.

BUSINESS
Alkem Labs sees huge sales momentum of anti-infectives in Q1FY22
Secondary infections due to bacteria and fungus have gained traction pushing up sales; healthy momentum in chronic therapies and increased usage of vitamins, nutrients and minerals act as growth triggers
BUSINESS
Zydus Cadila enters pact with Taiwanese drug maker TLC to distribute Amphotericin B injection in India
Under the terms of the agreement, TLC will manufacture and supply AmphoTLC on a non-exclusive basis to Zydus, and Zydus will commercialize the antifungal drug in India.
BUSINESS
Pfizer says hasn't authorised anyone to import, distribute vaccine in India
Pfizer tells Moneycontrol it is supplying vaccines only to national governments. The response comes a day after the Maharashtra government said it received bids from intermediaries that claimed to have tie-ups with Pfizer and other vaccine makers.

BUSINESS
Bharat Biotech says it expects Covaxin WHO emergency use listing in July-September
The vaccine maker also said that it is in the process of seeking regulatory approvals for Covaxin in more than 60 countries including US, Brazil, Hungary, among others.

INDIA
COVID-19: As vaccine shortage bites, Centre unlikely to take over procurement completely
With their procurement tenders getting tepid response from global pharma majors, more states now want the Centre to once again take over as the sole procurer of vaccines. The Centre is unlikely to do so even as a supply-side shortage is expected, at least for the next three months

BUSINESS
Bharat Biotech to expedite Covaxin WHO emergency listing with govt backing
Vaccine meets criteria laid out by the World Health Organization for issuing emergency use listing, and approval is expected in the second half of 2021; Company submitted EOI in April
BUSINESS
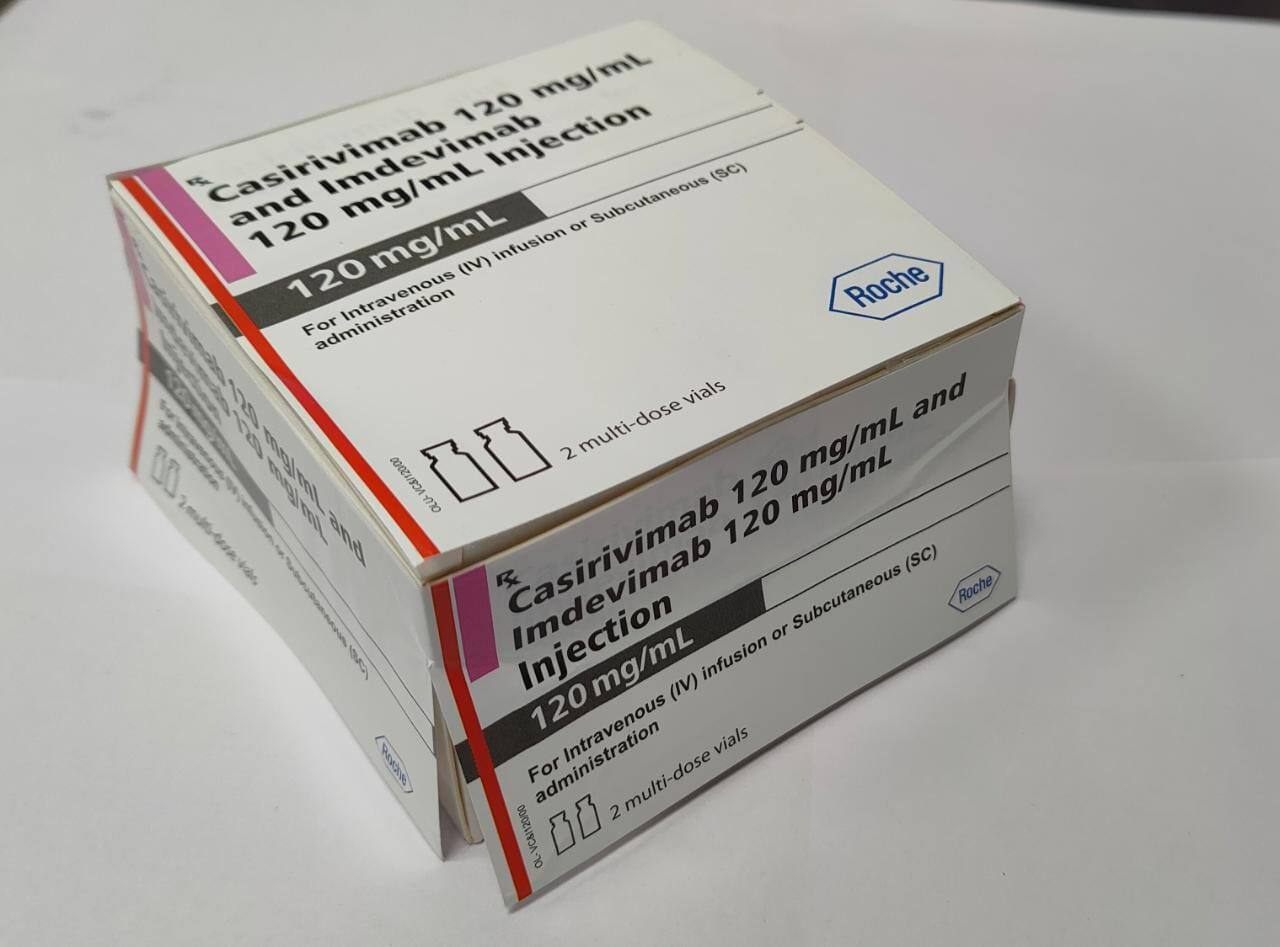
COVID-19 treatment: Roche, Cipla launch antibody cocktail Casirivimab and Imdevimab in India, costs Rs 59,750
The price for each patient dose [a combined dose of 1200 mg (600 mg of Casirivimab and 600 mg of Imdevimab) will be Rs 59,750 inclusive of all taxes.

BUSINESS
Govt to import 6.8 lakh vials of Amphotericin-B injections in May, June; domestic production ramped up to 2.6 lakh vials
The government said in May 2021, 3,63,000 vials of Amphotericin-B will be imported, thereby resulting in total availability in the country inclusive of the domestic production of 5,26,752 vials.

BUSINESS
Sun Pharma appoints Pawan Goenka, Rama Bijapurkar as additional independent directors
The board of directors of Sun Pharma approved the appointment of Goenka and Bijapurkar as additional independent directors on the board of the company, with effect from May 21, 2021, to hold office up to the ensuing 29th annual general meeting of the company, Sun Pharma said in a regulatory filing.
BUSINESS
Five pharma cos get permission to produce antifungal drug Amphotericin B liposomal injection
The five companies that got permission to manufacture are Natco Pharma, Gufic Biosciences, Alembic Pharmaceuticals, Emcure Pharmaceuticals and Lyca Pharmaceuticals.

BUSINESS
Chemists' body threatens to join lockdown, if its members are not vaccinated on priority
AIOCD said that since last year March 2020 till date, more than 650 Chemists and Pharmacists lost their lives due to COVID-19 infections.

INDIA
MBBS students, fresh graduates fill gaps in COVID wards, but there are challenges
Hospitals are deploying medicine students and freshly graduated doctors for COVID care, but many complain of long hours and delayed salaries at government facilities, while senior physicians get the additional burden of training raw talent.
BUSINESS
Optimus Pharma gets DCGI nod to conduct Phase-3 trial of COVID-19 antiviral drug Molnupiravir
Optimus Pharma has internally developed the active pharmaceutical ingredient and the formulations for the product it had filed for clinical trials with the DCGI.

BUSINESS
Pharma firms scramble to boost production of antifungal jab Amphotericin B as Black Fungus cases rise
Industry sources told Moneycontrol that it would take at least 15-30 days for new production to reach pharmacy shelves. The drug is complex to manufacture and would require a certain number of days of sterility data before the batch is released into the market.
BUSINESS
Commercial launch of anti-COVID drug 2-DG will be in June, price is being determined, says Dr Reddy's
"We expect commercial launch of the drug in June, and expect to supply it to hospitals," the spokesperson of Dr Reddy's said.
BUSINESS
Sputnik V vaccine update: Dr Reddy's says 8-9 states have approached company for procurement; announces tie-up with Apollo Hospitals
Dr Reddy's will use the Apollo Hospitals network nationally for storing, administering and monitoring the vaccination. The initial rollout will begin in Hyderabad on May 17 and will be extended to Visakhapatnam and other metro cities starting May 18.

BUSINESS
COVID-19 drug Baricitinib: Eli Lilly signs voluntary licensing agreement with Natco Pharma
Natco has received approval from the Drug Controller General of India (DCGI) for Baricitinib earlier in May. Natco went ahead and launched the drug under brand name Barinat even as its compulsory licensing application is still pending before India. The patents of Baricitinib are owned by Lilly.

BUSINESS
Hester Biosciences in discussions with Bharat Biotech for tech transfer of Covaxin
"A triparty consortium has been formed with the Government of Gujarat as the lead partner, to explore the prospects of manufacturing the Covid vaccine through technology from Bharat Biotech," Hester Biosciences said in a statement.

BUSINESS
COVID-19 | Dr Reddy's to launch 2-DG medication in June
"We have started the production of this product, we expect this product available from June," said Deepak Sapra, Senior Vice President and Head of Pharamceutical Service and Active Ingredients (PSAI), DRL.
BUSINESS
Dr Reddy's says it plans to vaccinate 125 million people with Sputnik V vaccine in next 8-12 months
"We have a supply committment of 36 million doses from RDIF in next two months," said M V Ramana, Executive Vice President and Head of the Branded Formulations at Dr. Reddy's.
BUSINESS
Explained: Will extending gap between two Covishield doses help save more people?
The government said it based its decision on the COVID Working Group, chaired by Dr NK Arora that reviewed the available real-life evidence before recommending the extension of the gap between the two doses.








