
BUSINESS
Emcure says it will continue growth momentum in FY22 by deepening product portfolio, digital focus
The privately held Pune-based company aims to first bring generic drugs to the market through the company’s own R&D efforts and innovative molecules through licensing

BUSINESS
Fortis says only 1% of its staff who tested COVID-19 positive post two doses required ICU/ventilator support
The study assessed around 16,000 healthcare workers with Fortis network who had been administered both first and second doses of COVID-19 vaccine between January 2021 and May 2021. After receiving both the doses, only 6 percent staff got infected.
BUSINESS
RDIF says Sputnik V will soon offer booster shot that's adjusted to work against Delta variant
Sputnik V uses heterogeneous boosting with 2 shots by 2 different adenoviral vectors Ad5 and Ad26. The adenoviral vector Ad26 is used for prime boost, and Ad5 is used as booster dose. RDIF didn't clarify which vector it would be adjusting against Delta variant.

BUSINESS
Widening Covishield gap - did the govt rely on proper evidence and follow due process?
The government has asserted that the prescribed gap between two doses was increased because real-life evidence, mainly from UK, suggested that this would increase efficacy, but UK itself reduced the gap, which triggered a controversy
BUSINESS
BMC orders inquiry into alleged fake vaccination drive at Mumbai's residential society
Beneficiaries generally show mild symptoms afterwards. However, no such symptoms were seen in this case.

BUSINESS
In final leg of Sputnik V pilot rollout, will announce commercial launch soon, says Dr Reddy's
"Being a limited pilot phase presently, registration on CoWIN is not open yet to members of the public. This will become open at the time of our commercial launch," the company said in a statement on Wednesday.
BUSINESS
Suven Life Sciences to spend $40 mn on US clinical trials of two molecules targeting Alzheimer's
Though SUVN-502 failed to meet a phase two study in the US, trial data has shown that the drug shows some promising results on agitational symptoms of Alzheimer’s patients. SUVN-G3031’s clinical trial is expected to be completed in a year and a half.

BUSINESS
COVID-19 | Thermo Fisher launches RT-PCR test system that can deliver results in 30 minutes
The Accula SARS-CoV-2 test has received Emergency Use Authorisation from the USFDA for thje Clinical Laboratory Improvement Amendments–waived environments.
BUSINESS
Explained | What Novavax data means to India, approval timelines, volumes and price
Will the Novavax COVID-19 vaccine offer protection against the deadly Delta variant of the coronavirus? How much will it cost in India? Find out answers to this and other questions about the vaccine that can be preserved in household refrigerators as well
BUSINESS
Bharat Biotech defends Covaxin high price in private market, says rate offered by govt 'not sustainable in long run'
Bharat Biotech said as per the direction of the Indian government, less than 10 percent of its total production of Covaxin to date has been supplied to private hospitals, while most of the remaining quantity was supplied to state and central governments. The weighted average price of Covaxin for all supplies realised by Bharat Biotech is less than Rs 250 per dose.

BUSINESS
Why Bharat Biotech's Covaxin remains expensive in private markets
A ready reckoner that seeks to explain why Bharat Biotech sells its COVID-19 vaccine sells its vaccine in the market at double the price of its competitor Covishield manufactured by Serum Institute

BUSINESS
Lupin gets UK nod to launch generic asthma inhaler drug Fostair
Respiratory diseases affect one in five people. It is the third biggest cause of death in the UK and is a clinical priority for the NHS.

BUSINESS
Lupin gets USFDA's warning letter for its Somerset facility in US
Lupin said in a statement to stock exchanges that it believed the letter won’t have any impact on supplies or existing revenues.

BUSINESS
USFDA recommendation to file full BLA for Covaxin could be a setback for Bharat Biotech, Ocugen
A new clinical trial would mean a stretch of timelines, and possibly delay by several months. The USFDA can take about six to 10 months to approve a Biologic Licensing Application.

BUSINESS
Additional clinical trial data needed for US approval of Covaxin: Bharat Biotech
Bharat Biotech didn't clarify the nature of the clinical trial - whether it is a local bridging trial or a Phase -3 trial sought by the USFDA. The vaccine maker said Covaxin has received emergency use authorisation’s from 14 countries with more than 50 countries in the process.

BUSINESS
Exclusive | Bharat Biotech working on a war footing to roll out Covaxin from Karnataka site
Malur production site expected to produce 30 million doses in July and 50 million by August. Currently, the entire production of Covaxin is done from its Genome Valley facilities on the outskirts of Hyderabad.
BUSINESS
Central govt's large order may compensate SII, Bharat Biotech for loss of high-priced states orders
A total of 440 million doses of COVID-19 vaccines from the two Indian manufacturers has already been placed; 30 percent advance for procurement of both the COVID vaccines has been released to the companies; from June 21, all citizens above the age of 18 years will get free vaccines

BUSINESS
Bharat Biotech to make Covaxin's Phase-3 results data public in July
The company added that once the data from final analysis of Phase III studies are available, Bharat Biotech will apply for full licensure for Covaxin

BUSINESS
DRDO invites applications from pharma companies for tech transfer of COVID drug 2-DG
DRDO has given time till June 17 for manufacturers to submit EoI. DRDO set a ToT fee of Rs 25 Lakhs.
BUSINESS
Glenmark says post-marketing study of COVID-19 antiviral Fabiflu supports the drug safety and effectiveness
"The time to fever resolution was seen on day 3, while two-third of the patients achieved clinical cure on day seven," Glenmark said.
INDIA
Free COVID-19 vaccines: Centre to provide 25% jabs to states based on population, disease burden, inoculation progress
States and UTs would aggregate the demand of private hospitals keeping in view equitable distribution between large and small private hospitals and regional balance.
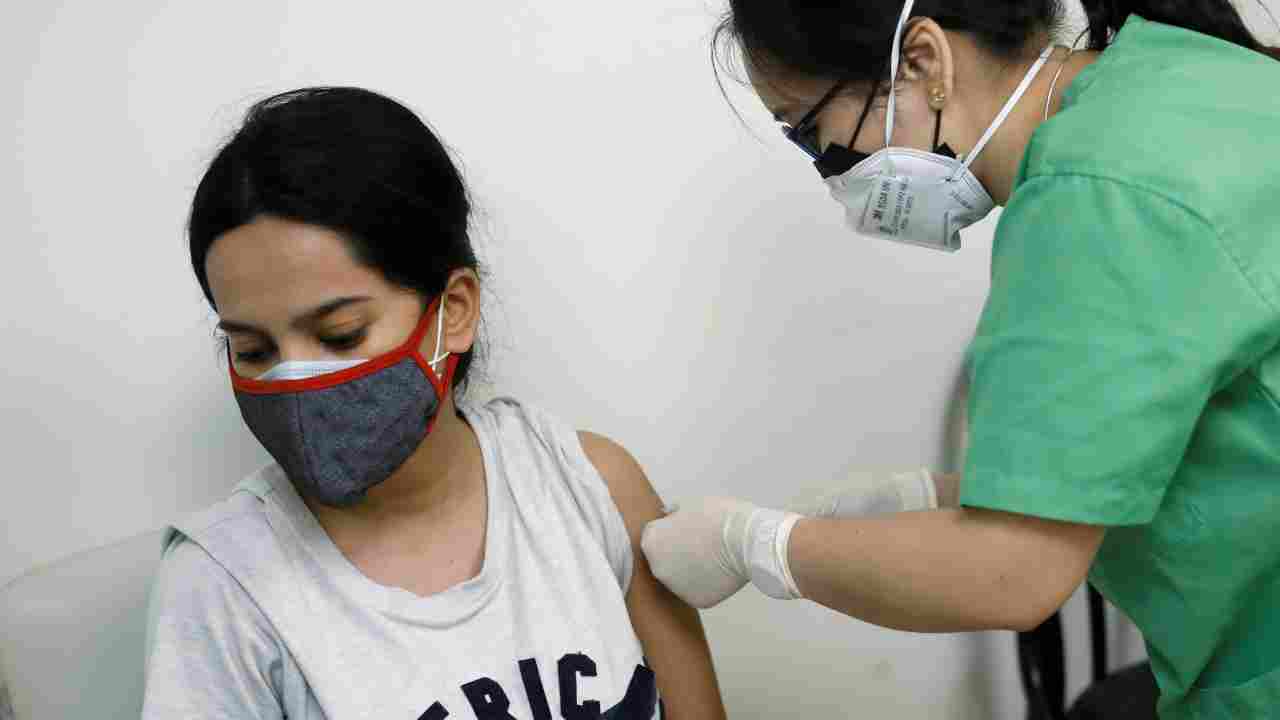
BUSINESS
Private hospitals divided on Rs 150 cap on COVID-19 vaccine administration charges
Corporate hospital chains such as Apollo, Max Healthcare, Fortis Healthcare, Narayana Health and Manipal Hospitals have shown huge interest in offering COVID-19 vaccination.

BUSINESS
Explained | What is indemnity and why vaccine manufacturers are demanding it in supply contracts
Legal and public health experts are calling the government to be more transparent on such arrangements, and develop a mechanism for compensation for vaccine related injury and death; in case the government decides to provide indemnity. They also demand strengthening of adverse event following immunisation (AEFI) reporting and investigations.

BUSINESS
Is the govt taking a risk on Bio E vaccine that's yet to cross the finish line?
The government’s Rs 1,500 crore bet on Biological E’s vaccine candidate that is still undergoing trials may be a risk worth taking, experts said but an industry executive said the money should have been used to buy vaccines already in the market








