
BUSINESS
Cipla seeks price hike for certain respiratory medications, writes to NPPA
Cipla spokesperson confirmed the development, and said the products impacted are a "small subset" of the overall basket of respiratory products.
BUSINESS
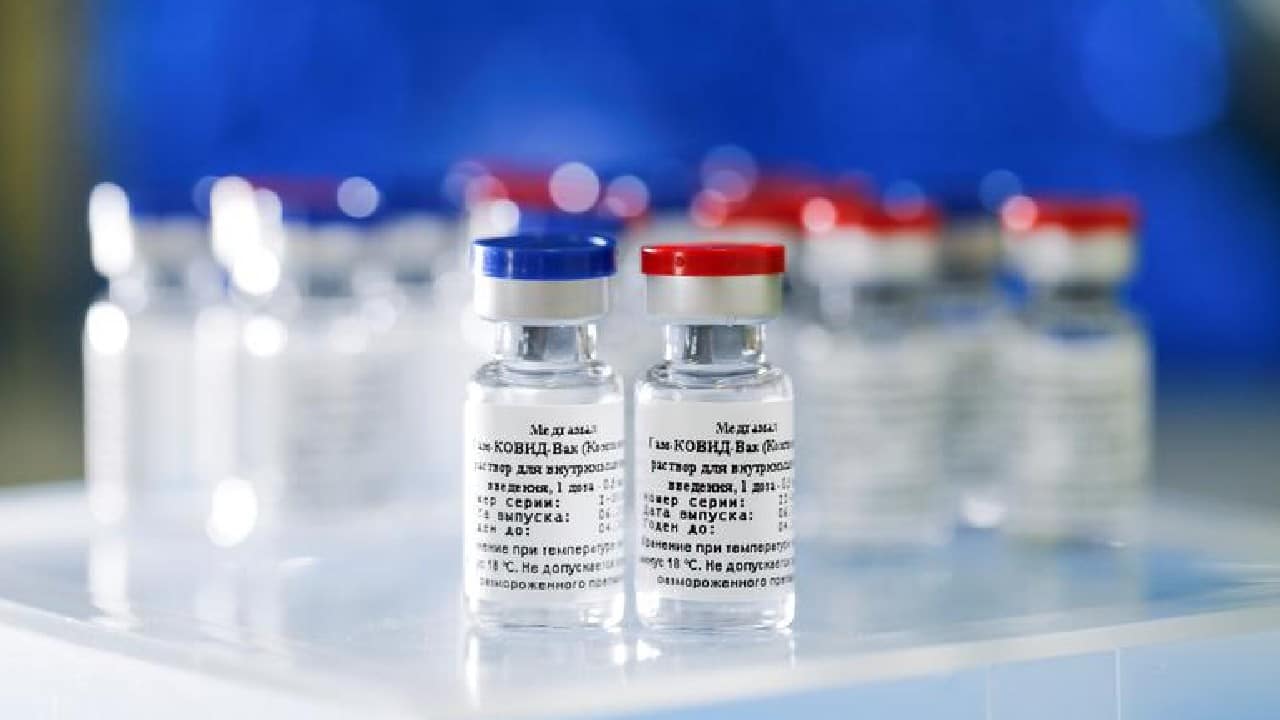
Panacea Biotec rolls out first batch of Sputnik V second jab
The second jab of Sputnik V is being manufactured at the Panacea Biotec’s facility in Himachal Pradesh

BUSINESS
Goldman Sachs-backed Aragen Life Sciences on expansion mode, evaluating select acquisition opportunities
With revenue of Rs 1,200 crore in the financial year 2021, Aragen provides outsourcing services for drug discovery, clinical trials, and manufacturing to over 450 clients globally, including many large biotech and pharmaceutical companies.
BUSINESS
Hetero to launch Tocilizumab, gets DCGI’s emergency use nod
While Hetero didn't announce the price of the drug, but the biosimilar version is expected to be cheaper than the branded Tocilizumab which is priced at Rs 40,600 per single injection. A COVID-19 patient would typically require about two injections.
BUSINESS
Govt utilised 23.6% of budgeted Rs 35,000 crore for COVID-19 vaccines till July
The government has spent Rs 9,229.31 crore between March and July to procure 124 crore doses from three manufacturers.

BUSINESS
SII puts Indian demand for COVID-19 vaccine above exports
"Once the supply-demand situation eases in India, we will think about exports," said one of the people, requesting anonymity.
BUSINESS
New list of essential medicines: 39 new drugs added, 16 dropped
Most of the new drugs added to the list were anti-cancer like Azacitidine and Fludarabine antiretroviral such as Dolutegravir, Darunavir+Ritonavir, new generation TB medications such as Bedaquiline and Delamanid, anti-allergy Montelukast, anti diabetes drugs like Teneligliptin and Insulin Glargine.

BUSINESS
Exclusive | SII, Bharat Biotech made additional 40-50 million doses of COVID-19 vaccine available in August
Increased vaccine supplies have helped the government to accelerate its inoculation drive

BUSINESS
Here is how Bombay Hemp Company is trying to create business out of Cannabis
Bombay Hemp Company or BOHECO was founded with a plan to create a vertically integrated company that produces hemp to make textiles, building materials, Ayurvedic remedies, and health and wellness products.

BUSINESS
Veeda Clinical Research filing for Rs 500 crore - Rs 700 crore IPO, sources say
The company sees increased traction in its core segment of studies of evaluating the generic versions of proprietary drugs that go off patent, as well bright prospects from clinical trials of biosimilars and novel drugs.

BUSINESS
Bharat Biotech on target to reach a billion doses, rolls out Covaxin from Ankleshwar unit
With specialised bio safety containment facilities in Hyderabad, Malur, Ankleshwar, and Pune, Bharat Biotech said it is steadily moving towards its aim of ~ 1 billion doses of annualised capacity.
BUSINESS
IPO-bound Ami Organics confident of growing business despite Chinese competition
The company has been able to gain significant market share on select intermediates over the years despite tough competition from Chinese suppliers, that dominate intermediate and APIs globally.

BUSINESS
IPO-bound Vijaya Diagnostic looks to deepen presence in AP, Telangana and East India
Vijaya Diagnostic will be the second IPO of a diagnostic lab chain to hit the market in less than a month. Earlier this month Krsnaa Diagnostics IPO was subscribed 64.40 times. The price band of the offer has been fixed at Rs 522 to Rs 531 per equity share.

BUSINESS
Cipla partners Kemwell to foray into respiratory biosimilars space
Cipla’s respiratory drug prowess combined with Kemwell’s expertise in biologics will accelerate bringing these essential products to market.

BUSINESS
J&J says COVID-19 booster jab generates strong antibody response
The interim data from new studies shows that a booster dose generated a rapid and robust increase in spike-binding antibodies, nine-fold higher than 28 days after the primary single-dose vaccination, J&J has said
BUSINESS
India's Covid vaccination drive set to gain pace from September with steady supplies
Monthly vaccine manufacturing capacity is to rise to around 360 million doses by the year-end from 130 million doses now, says a CARE Ratings report. At least 2 billion doses more are needed to cover the entire population
BUSINESS
Bharat Biotech's Chikungunya vaccine: Phase 2/3 trial begins
The Global Chikungunya Vaccine Clinical Development Program (GCCDP) seeks to develop and manufacture an affordable Chikungunya vaccine with the aim of achieving WHO prequalification to enable its distribution in low- and middle-income countries.

BUSINESS
Hester Biosciences says on track to produce Covaxin drug substance by Q4FY22
The construction of a BSL-3 facility suitable for manufacturing the drug substance of Covaxin is underway. The vaccine maker intends to produce 5 million to 15 million doses of Covaxin drug substance.

BUSINESS
Gennova gets DCGI nod to conduct Phase 2,3 trials of its mRNA COVID-19 vaccine
The study will be conducted in India at 10-15 sites in Phase II and 22-27 sites in Phase III. Gennova plans to use the DBT-ICMR clinical trial network sites for the study.

BUSINESS
Zydus expects Rs 200-250 crore monthly sales of ZyCoV-D vaccine from October quarter
The company said it is in discussions with the government on pricing and volumes of the needle-free COVID vaccine and expects to roll out one crore doses per month from October.
BUSINESS
Aurobindo Pharma faces delay in getting its COVID-19 vaccine plan rolling
Aurobindo Pharma has exclusive rights to develop and commercialise UB-612 in India and to UNICEF, and non-exclusive rights in other select emerging markets. The company can supply up to 480 million doses of UB-612 for India and other countries.

BUSINESS
Zydus Cadila to launch ZyCoV-D in September, clarity on pricing in 1-2 weeks
Patel said the company is targeting to supply 4 crore doses of ZyCoV-D by end of December, and possibly 5 crore by end of January. Zydus has capacity to manufacture 10-12 crore doses of ZyCoV-D annually.

BUSINESS
DCGI approves Cadila Healthcare's ZyCoV-D COVID vaccine for all above 12 years of age
The vaccine has to be administered in three doses – the first dose, and the remaining doses after 28 and 56 days.

BUSINESS
Explainer: All you need to know about ZyCoV-D, the indigenous plasmid DNA COVID vaccine
The three-dose vaccine is delivered through a needle-free applicator called the PharmaJet to ensure painless intradermal vaccine delivery. The intradermal route (between the layers of the skin), makes its administration much easier. DNA vaccines are also theoretically easy to redesign quickly against a mutating virus.









