



The crackdown on exports of substandard cough syrup from India is impacting all exporters. The government testing facilities that analyse cough syrups meant for exports are seeing a pendency of samples, with at least two of the seven designated labs in the country having more than 100 cough syrups under testing.
According to sources, the pending cough syrup samples under testing in the government laboratories and the delay in clearance has forced the manufacturers to seek alternative mechanisms for analysis from the drug regulator.
“The government has taken note of the problem faced by the exporters. The National Accreditation Board for Testing and Calibration Laboratories (NABL) has been asked to prepare a list of private testing laboratories where the cough syrup samples can be sent for analysis,” an official aware of the development told Moneycontrol.
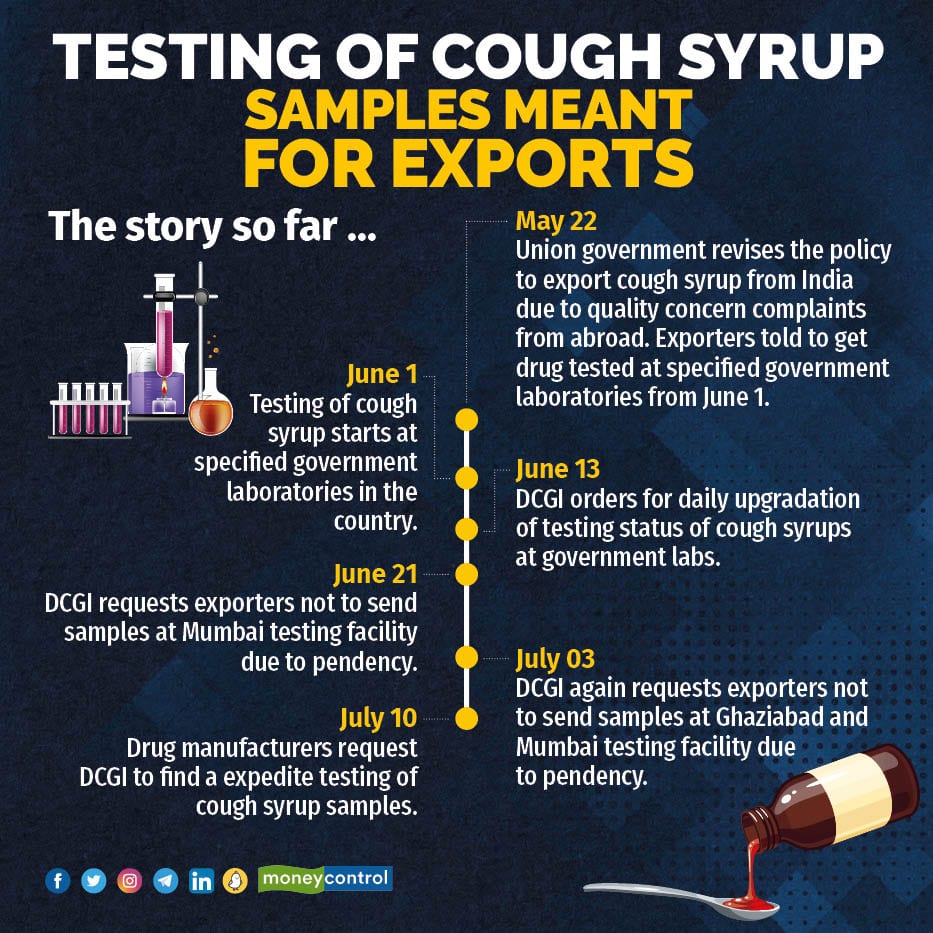
The Union government on May 22 amended the cough syrup export policy to curb substandard drug supply.
The amended policyAs per the amended policy, the pharma companies from June 1 were allowed to export cough syrups only after they produce the certificate of analysis (CoA) from any of the six central, regional drug testing laboratories or any NABL accredited laboratories.
According to another official, analysis of a cough syrup to detect Di-Ethylene Glycol (DEG) and Ethylene Glycol (EG) contamination can take a fortnight.
When asked if the delay in sample analysis was also resulting in a delay in exports, the official said the effect was “minuscule”.
The demand for roping in private testing facilities for analysis of cough syrup came after Rajeev Singh Raghuvanshi, Drug Controller General of India (DCGI), in two separate communications to drug exporters, seen by Moneycontrol, informed about the pendency of more than 100 cough syrups for analysis in Ghaziabad and Mumbai testing facilities, requesting them not to send samples in these labs.
“Based on the data of July 3, it is observed that IPC Ghaziabad and CDTL Mumbai are having more than 100 cough syrups under testing and some of the labs are not having any samples for testing,” DCGI said in the letter.
Raghuvanshi in the letter requested the manufacturers and exporters not to submit any samples in Ghaziabad and Mumbai testing facility, till all the samples are analysed and reports are released.
“Manufacturers and exporters are requested to verify the website information of CDSCO on a daily basis and submit the samples at the laboratories which are having no or less number of samples,” the letter said.
Also read: Mandaviya bats for self-regulation by small pharma firms, says won’t compromise on drug quality
The Indian Drug Manufacturer Association (IDMA) without giving details said the government was working to solve the problem.
“We have discussed this issue with the DCGI and have been informed that an alternative is being worked out to solve the issue of pendency of samples for analysis,” Viranchi Shah, National President, IDMA said.
The NABL officials did not respond to Moneycontrol queries on the criteria for engaging private accredited labs for testing the samples.
‘Testing before exports is a cosmetic approach’Terming the whole exercise of testing a few samples from a batch before export as a "cosmetic approach", Ravi Uday Bhaskar, Director General, Pharmexcil, a government body looking after pharma exports, said the Indian regulator needs to do a root-cause analysis of the shortcomings in drug manufacturing.
Also read: Quality the key to transform Indian Pharma
“The regulator needs to send a message to the unscrupulous elements,” he added.
Bhaskar, who suspended the export registration of three companies involved in exporting adulterated drugs, said instead of testing cough syrup samples the regulator should focus on the implementation of Good Manufacturing Practices (GMP) in drug plants.
“The onus of producing quality medicine by complying with the guidelines should be done by the manufacturer on its own, why should government testing laboratories be engaged for this? Tomorrow if a manufacturer sends a different sample for testing and exports a contaminated product, then our testing labs will be in the line of fire,” he added.
Discover the latest Business News, Sensex, and Nifty updates. Obtain Personal Finance insights, tax queries, and expert opinions on Moneycontrol or download the Moneycontrol App to stay updated!
Find the best of Al News in one place, specially curated for you every weekend.
Stay on top of the latest tech trends and biggest startup news.