



With the World Health Organisation’s (WHO) probe into drug adulteration flagging Indian products for adulteration or substandard medicines, the office of the Drug Controller General of India has now issued a slew of show-cause notices, with 45 ‘vulnerable’ manufacturing sites put on watch in Himachal Pradesh alone after carrying out a ‘risk-based inspection’ at plants of different firms.
Officials collected data on drugs found to be ‘not of standard quality’ (NSQ), adulterated or spurious between 2019 and 2022, along with the name of manufacturing units from state regulators.
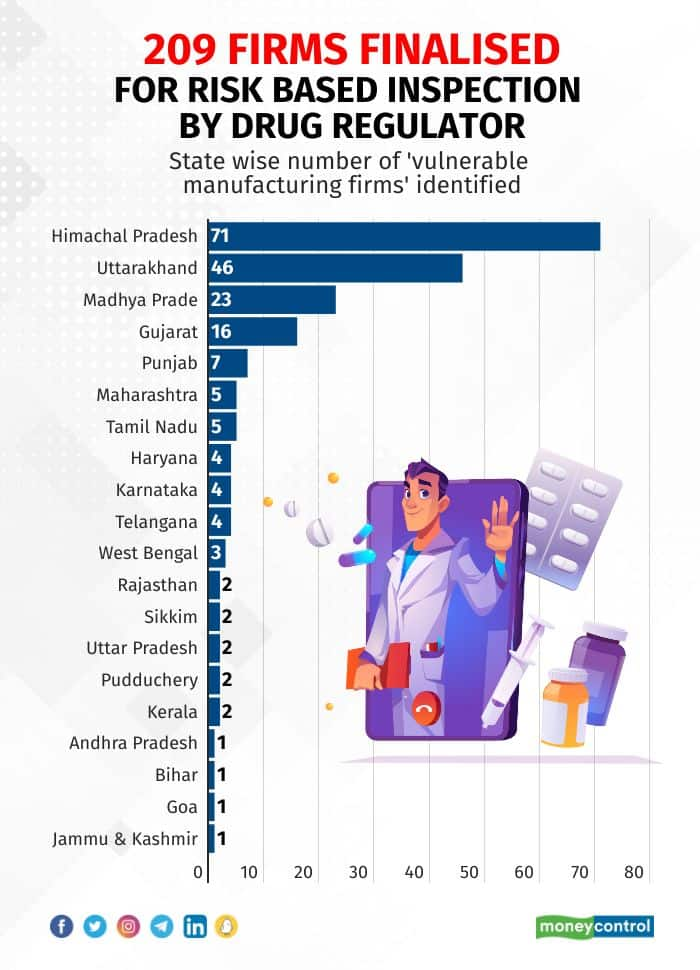
According to the data accessed by Moneycontrol, the regulators identified 209 pharmaceutical firms, each having more than 11 NSQ warnings.
Also read: Marion Biotech's licence to be cancelled after contamination confirmed
The regulators, after inspecting 51 firms in Himachal Pradesh, the most in any state, have issued notices to 26 manufacturing units in the hill state.
In India, Himachal Pradesh is a pharmaceutical hub having almost 1,500 functional units. The Baddi Barotiwala Nalagarh (BBN) belt and Paonta Sahib are most preferred locations for pharma manufacturing.
“16 manufacturing companies have been given the SPO (stop production order) and 2 have been given the notice of firm cancellation,” the government document said.
Also read: Maiden Pharma’s cough syrups from Himachal plant flagged for adulteration
Officials said that the drug regulator so far has conducted three risk-based inspections on 137 erring firms.
In Phase 1, 78 firms, each with 20 and above NSQs out of 209 firms, were selected for joint inspection by the zonal office of the Central Drugs Standard Control Organisation and state licensing authorities. For Phase 2, the next 44 firms, each with 15 to 19 NSQs selected based on intelligence inputs, were selected and three additional firms were selected based on complaints.
In Phase 3, the next 12 firms were inspected, each with 11 to 15 NSQs. The officials, however, didn’t share the names of the firms identified for the inspection.
WHO names India in contaminated drug probeThe WHO in its probe of contaminated cough syrup supply flagged 20 products exported from two countries, India and Indonesia.
“To date this situation has impacted more than 20 products with two countries of origin (India and Indonesia) and more than 15 different manufacturers. For the date, this refers to the number increasing over time. So it is approximately 15 manufacturers,” WHO spokesperson Christian Lindmeier told Moneycontrol in an email response.
Also read: Govt tweaks policy to prevent export of spurious cough syrup
When asked about the details of the manufacturers involved in exports of substandard drugs, Lindmeier said the WHO can’t divulge names or details of manufacturers other than those mentioned in the medical alerts.
“Best to contact the authorities in India to understand the details of the investigation they may be doing,” he added.
The WHO spokesperson further said that the global body was aware of media reporting of potentially contaminated syrups in other countries.
“Whilst investigations are ongoing, WHO has not expanded its list of medical product alerts. Our investigations with the impacted countries are ongoing. To date, we cannot confirm a link. This may change as we receive more information,” he added.
Discover the latest Business News, Sensex, and Nifty updates. Obtain Personal Finance insights, tax queries, and expert opinions on Moneycontrol or download the Moneycontrol App to stay updated!
Find the best of Al News in one place, specially curated for you every weekend.
Stay on top of the latest tech trends and biggest startup news.