BUSINESS
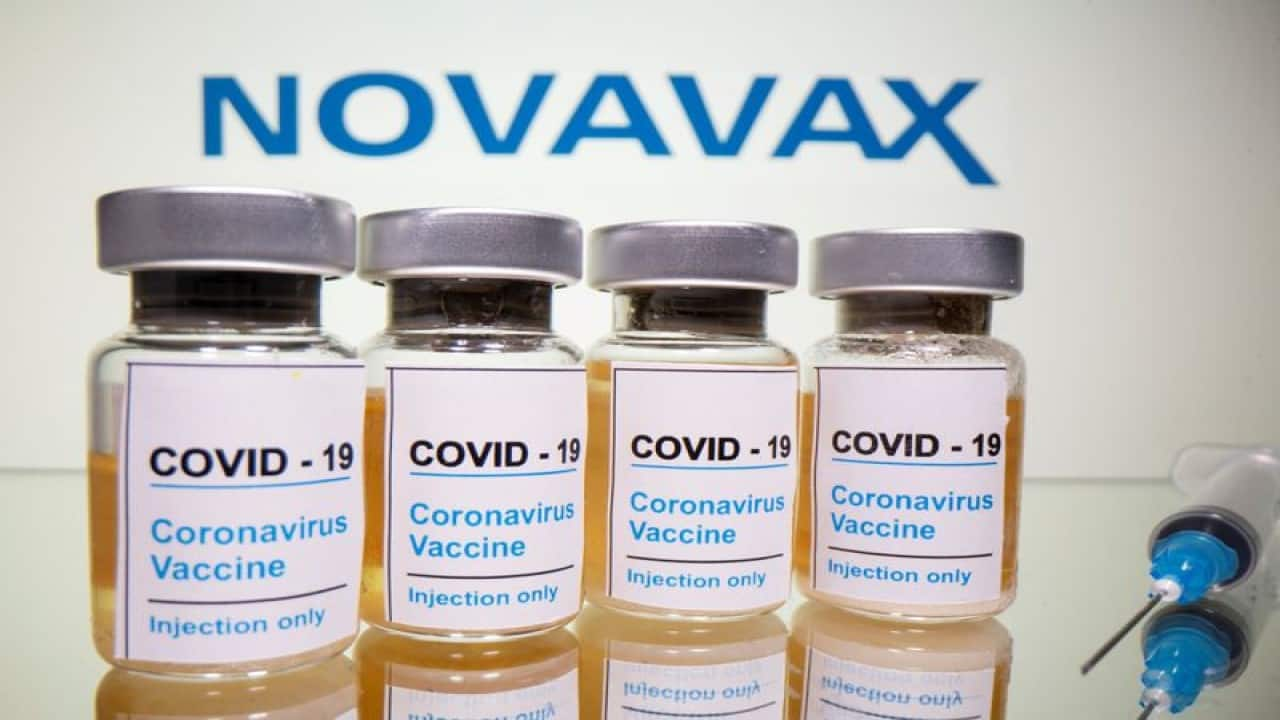
CDSCO panel allows Serum Institute of India to conduct bridge trial of Novavax COVID-19 vaccine
Novavax had earlier licensed its COVID-19 vaccine technology to SII with no upfront, milestone, or technology transfer payments.
BUSINESS
Explained | Why kids need separate trials for COVID-19 vaccine, how are trials conducted, and enrollment challenges
While US firm Moderna has started testing its vaccine on children, Pfizer and AstraZeneca-Oxford University are planning to follow suit. In India, Bharat Biotech is seeking permission to test its vaccine, Covaxin, on children aged between 5 and 18 years.

BUSINESS
COVID-19 vaccines: Public health experts urge government to release details of adverse event data
Twenty-nine experts from various fields have written a letter noting that the government had stopped sharing any details on Adverse Events Following Immunisation (AEFI) after February 26, 2021. The experts have sought details from the government on the findings of all completed AEFI investigations. The letter said that at least 65 deaths have occurred following vaccination for Covid-19 since the campaign began on January 16. However, the National AEFI Committee’s reports on only two of those deaths have been made public.
BUSINESS
Gland Pharma teams up with RDIF to manufacture 252 mn doses of Russia's Sputnik V COVID vaccine
Under the terms of the agreement, Gland Pharma said it will first undertake technology transfer of the drug substance to its manufacturing facilities. After successful technology transfer, Gland Pharma will then undertake manufacturing of drug substance and drug product filling into vials under aseptic conditions.

BUSINESS
Will White House backing hoist Biological E to the big league of COVID-19 vaccine makers?
After being overshadowed by the Serum Institute of India and Bharat BioTech, India’s first private vaccine maker has sprung into action with US funding that will help it produce 1 billion doses of COVID-19 vaccines by the end of next year.

BUSINESS
No evidence of link between COVID-19 vaccine, blood clots: AstraZeneca
AstraZeneca said there were no confirmed issues related to any batch of its vaccine used across Europe, or the rest of the world.

BUSINESS
Pharma wrap | AstraZeneca-Oxford vaccine on hold in Europe, what does it mean for India?
The Indian government has said it is reviewing the situation. The vaccine hasn't been linked to any deaths in the country.

BUSINESS
Novavax COVID-19 vaccine found to be 96.4% effective in final analysis of UK Phase-3 study
The vaccine would be manufactured at eight locations including the Serum Institute of India, with which Novavax has an agreement to produce around 2 billion doses a year.

BUSINESS
Input material shortages may hit COVID-19 vaccine manufacturing, says report
The shortages may emerge though several suppliers expanded their capacity by 50 percent in 2020. Many are also planning to expand production by another 50 percent in 2021.

BUSINESS
DCGI drops 'clinical trial mode' condition for Bharat Biotech's Covaxin
Currently, those receiving Bharat Biotech’s Covaxin are asked to sign a consent form before being vaccinated.
BUSINESS
HMD bags order to supply 265 million auto disposable syringes from government
The government has issued tenders to procure 350 million pieces of AD syringes to use in COVID-19 vaccination.

BUSINESS
Covaxin or Covishield? What you should know about each COVID-19 vaccine
Granted people in India cannot choose between Serum Institute of India’s (SII) Covishield and Bharat Biotech's Covaxin but here is a guide to help you understand some of the important facts about each vaccine.

BUSINESS
Max Healthcare raises Rs 1,200 through QIP, sells shares at Rs 195.4 apiece
Max Healthcare proposes to utilise the net proceeds for meeting the capital expenditure and working capital requirements, including expansion of capacity, and increasing stake in existing and future subsidiaries.
BUSINESS
World may see 18 percent shortfall in supply of COVID-19 vaccines in 2021: Report
Airfinity pegs the global demand for COVID-19 vaccines in 2021 at 11.5 billion doses, while the forecasted production is estimated to be 9.5 billion doses.

BUSINESS
Brazil's drug regulator Anvisa inspects Bharat Biotech's facility, may give nod to Covaxin soon
Brazil is the first country to sign a definitive supply agreement with Bharat Biotech to procure 20 million doses of Covaxin on February 26. The deliveries are expected to begin during Q2 and Q3 of 2021.

BUSINESS
How doctor-turned-entrepreneur Chaitra Harsha wants to build India's next big CRO from Mysore
Dr Chaitra Harsha has dabbled in setting up dental clinics, a medical tourism venture, offered consulting services in the area of bioinformatics, among others.

BUSINESS
Why pharma sales function has too few women?
Though the marketing and sales division has traditionally given pharma sector its leaders, women are still a small percentage here. A pharma association data says that while the average percentage of women in most sectors in India is between 15- 35%, in the pharma industry, it is 10–15%.
BUSINESS
Pharma wrap: Has the reboot helped PM's Jan Aushadhi scheme to sell affordable medicines?
Plagued by supply chain and quality issues and corruption, the scheme was revamped as Pradhan Mantri Bhartiya Janaushadhi Pariyojana in 2015-16. The store count has increased from 5,140 in FY 2019 to 7,500 in FY21 and turnover has jumped from Rs 315 crore to Rs 600 crore.
BUSINESS
US export curbs on COVID-19 vaccine ingredients limiting efforts to scale up production: Adar Poonawalla
The US has invoked the Defense Act, which has a sub- clause that prevents the export of critical raw materials required for local vaccine manufacturers.

BUSINESS
EXPLAINED | Covaxin shows 81% efficacy, here's how it stacks up against Covishield
Here is a comparison between the two COVID-19 vaccines – Covishield and Covaxin - approved in India.

BUSINESS
81% interim efficacy data boosts Bharat Biotech's hopes of Covaxin exports
Bharat Biotech said another interim analysis and a final one is expected in the weeks ahead, which will give a more robust picture of Covaxin's efficacy.

BUSINESS
Govt steps up COVID-19 vaccination pace, to allow more private hospitals to administer vaccines
The government's latest direction to states, will significantly increase the capacity. India has a total of 43,486 private hospitals. On an average, India is inoculating about 5-6 lakh people a day, way short of the government's target of 13 lakh.
BUSINESS
COVID-19 vaccination | Private hospitals ramp up capacities to fast-track inoculation
It is estimated that 20,000 private facilities will be available as COVID-19 vaccination centre.

BUSINESS
About 92% people who tried using Co-WIN portal faced difficulties: Survey
In a separate survey Local Circles said it also found that the Prime Minister Narendra Modi taking the jab, helped to reduce the vaccine hesitancy to 36 percent.










