


Shares of Glenmark Pharma tumbled 3 percent in the early trade Tuesday on the back of observations issued by USFDA.
The US Food and Drug Administration (USFDA) has issued 7 observations to company's Baddi units as the company has failed to thoroughly review any unexplained discrepancy and also failure of a batch.
The observations includes, lack of written procedures for production & process controls and complaint records are deficient.
USFDA also mentioned that the records have not maintained so data can be reviewed annually to evaluate quality standards.
It also includes responsibilities & procedures applicable to quality control unit not fully followed, appropriate controls not exercised over computers/related systems and lack of employee training.
The USFDA had inspected Baddi unit from November 6-11.
The Baddi unit manufactures finished dosages and contributing less than 10 percent of total Glenmark’s US sales.
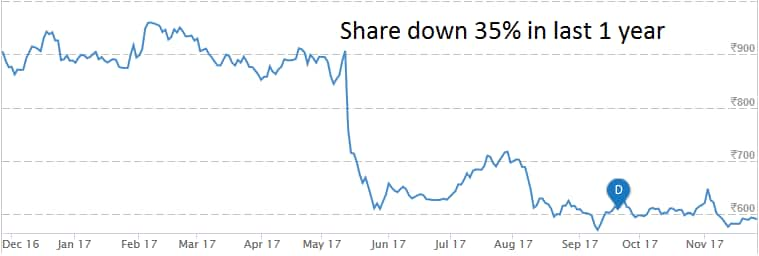
At 09:17 hrs Glenmark Pharma was quoting at Rs 578, down Rs 13.20, or 2.23 percent on the BSE.
Posted by Rakesh Patil
Discover the latest Business News, Sensex, and Nifty updates. Obtain Personal Finance insights, tax queries, and expert opinions on Moneycontrol or download the Moneycontrol App to stay updated!