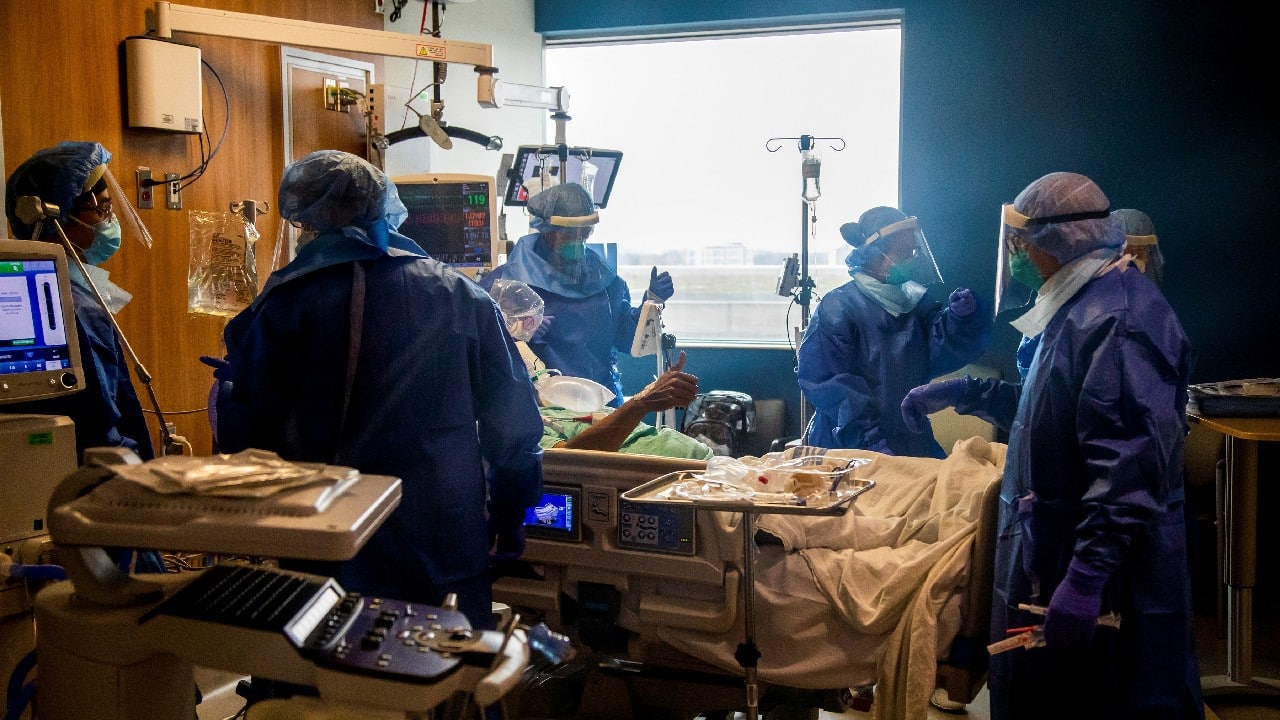
BUSINESS
Kerala's latest COVID-19 surge possibly linked to UK variant: INSACOG data
The UK variant which constituted just 1.06 percent cases on January 20 this year and 3.8 percent on March 20, now constitute 30.48 percent, as per data from April 25. In just one month the UK variant has grown almost 10 times.

BUSINESS
VINS Bioproducts begins human trials of horse antisera therapy to treat COVID-19 patients
The therapeutic product called VINCOV-19 is obtained after immunisation of horses with spike glycoprotein of the inactivated COVID virus.

BUSINESS
COVID-19 treatment | Natco Pharma seeks approval to launch phase-3 trial of Molnupiravir
Natco said it is hoping that CDSCO would give emergency approval of this drug based on “compassionate use” for patients.
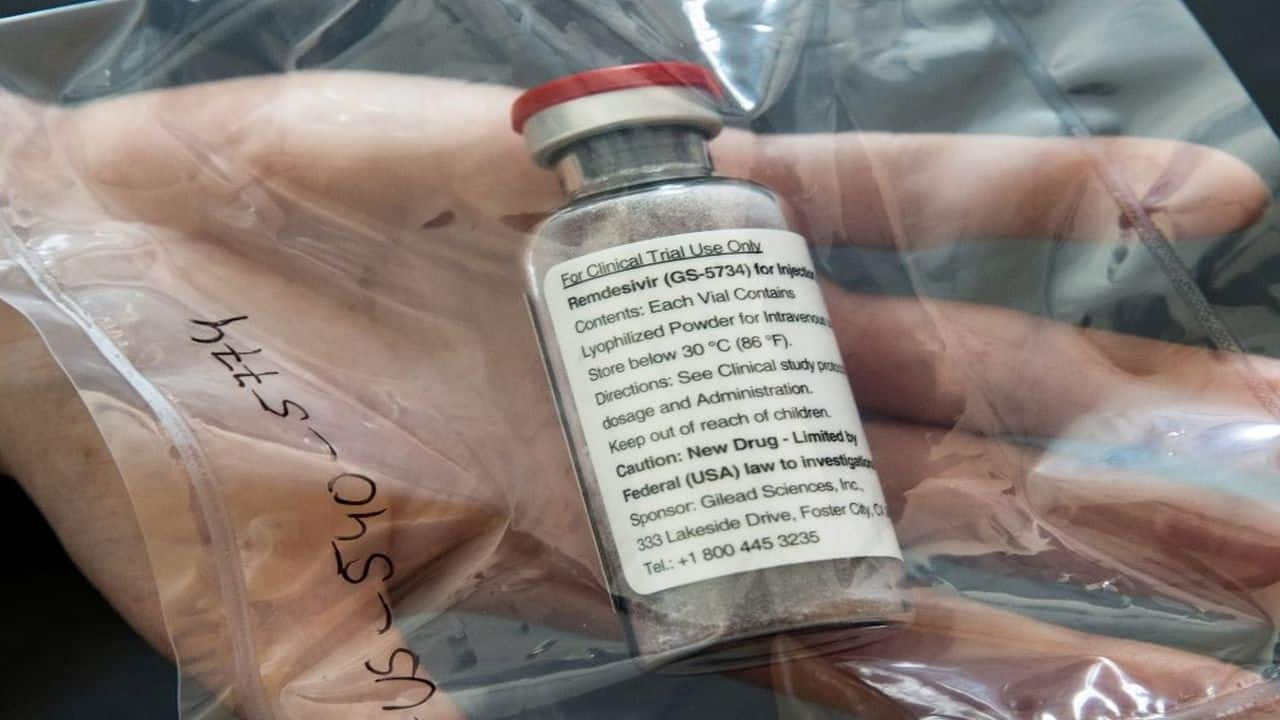
BUSINESS
Exclusive: Gilead to send Remdesivir vials to India, US stands by India to help fight COVID-19 2nd wave
Moneycontrol learns from sources that the Drug Controller General of India (DCGI) will have to issue an import license to bring the Gilead's Remdesivir stocks to India. The volume of this is still not known, but it will be in order of a few lakh vials.

BUSINESS
Smokers, vegetarians may be less likely to contract COVID-19: CSIR survey
The study assessed 10,427 adult individuals working in more than 40 CSIR laboratories and centers in urban and semi-urban settings spread across and their family members.
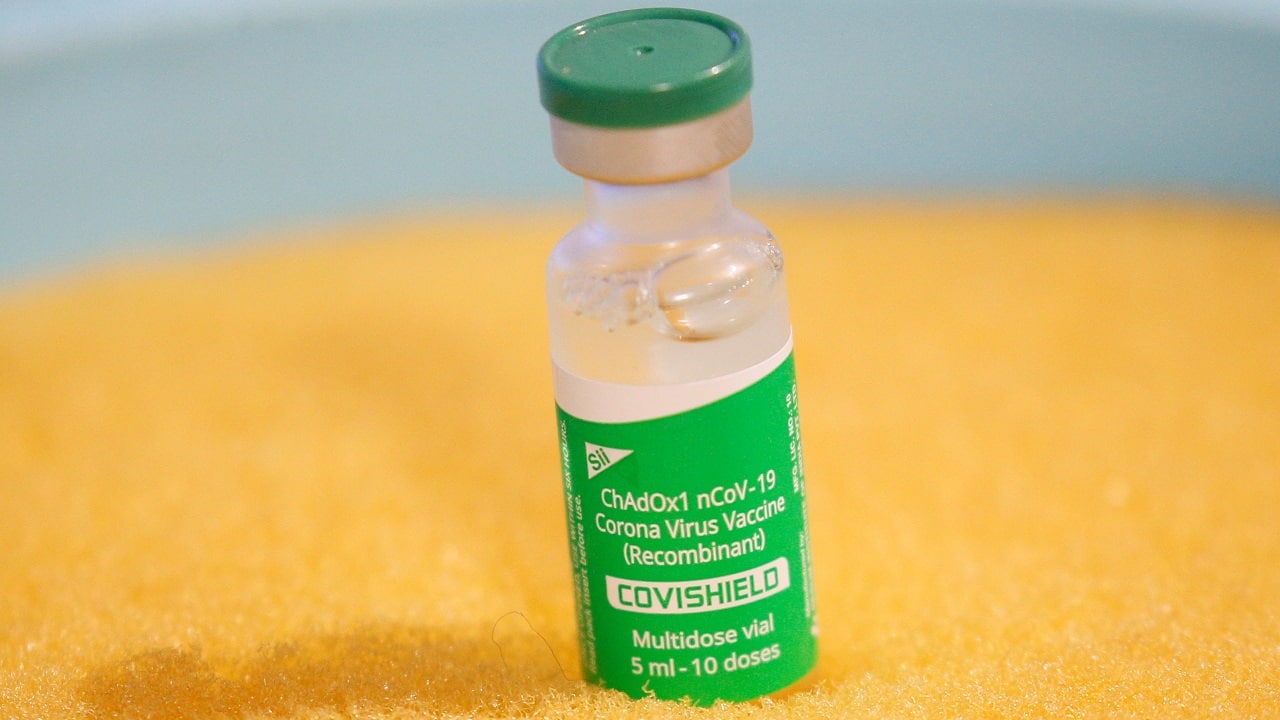
BUSINESS
Pharma wrap: COVID-19 vaccines in private market may cost Rs 900-1,500 per dose
Many states have already said they will offer COVID-19 free of cost to their citizens.

BUSINESS
Biological E gets nod to conduct late-stage clinical trials of its COVID-19 vaccine
The Phase III clinical study, which is to be conducted in 15 sites across India, will evaluate the Immunogenicity and Safety of Biological E’s SARS-CoV-2 COVID-19 vaccine for protection against COVID-19 disease in about 1,268 healthy subjects in the age range of 18 to 80 years. It is intended to be part of a larger global Phase III study.
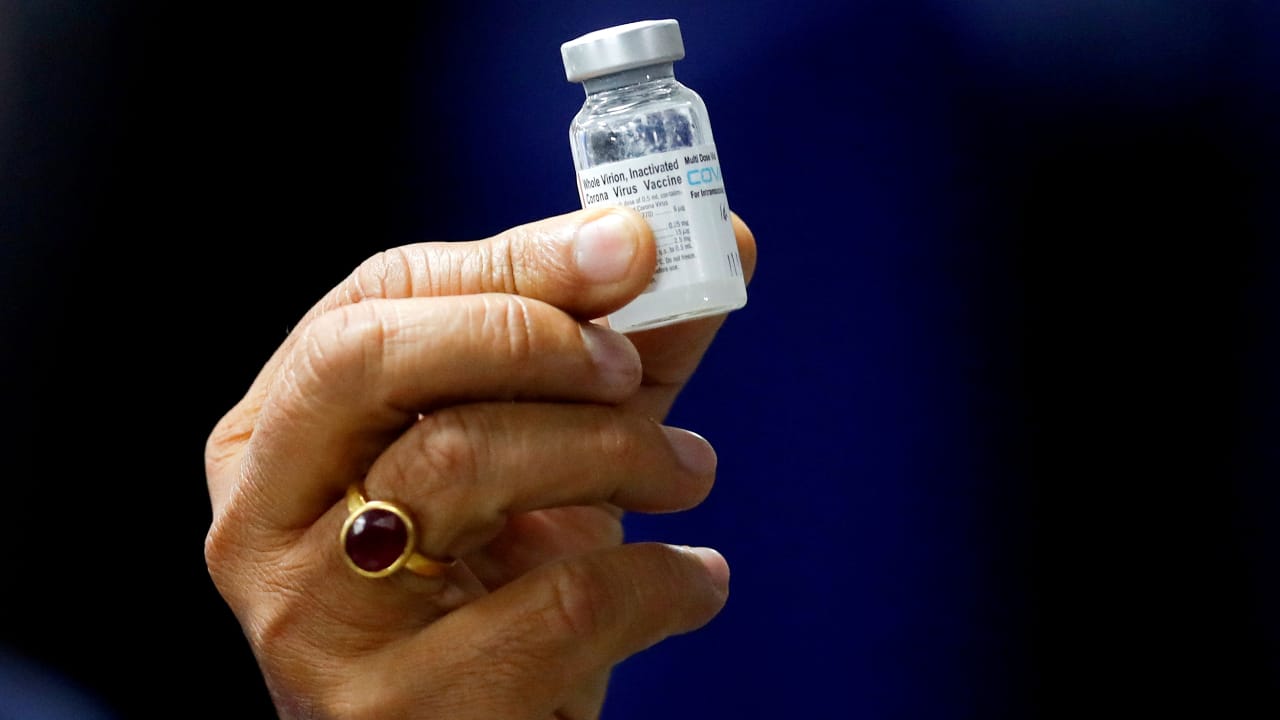
BUSINESS
Explainer | Covaxin, Covishield offering protection against double mutant confirmed: How scientists figured about the vaccines
The two announcements by government research institutions come as a relief, as scientists feared that the double mutant or B.1.617 strain of SARS-CoV-2 virus may dodge the immune system and render vaccines ineffective.

BUSINESS
Zydus Cadila’s Covid-19 vaccine: How it compares with peers, manufacturing capacities and challenges, explained
Zydus Cadila says its ZyCoV-D vaccine had very good results in Phase 1 and 2 trials. Dosing of participants in Phase 3 has been completed and the data analysis process is underway. However, not a single vaccine using its unique platform has been approved anywhere in the world. ZyCoV-D is also a three-dose vaccine, which could post logistical and administration challenges and weigh on pricing.
BUSINESS
Pfizer offers to supply its COVID-19 vaccine to Indian govt at not-for-profit price
The company has adopted a tiered price structure for its COVID-19 vaccine.
BUSINESS
Mankind Pharma in talks to distribute Sputnik V, says open to pacts for other COVID-19 vaccines
The Drug Controller General of India on April 12 approved the emergency use authorisation of Russia's Sputnik V COVID-19 vaccine.

BUSINESS
Bharat Biotech Covaxin demonstrates 78% efficacy in Phase-3 interim analysis
Due to the recent surge in cases, 127 symptomatic cases were recorded, resulting in a point estimate of vaccine efficacy of 78 percent against mild, moderate, and severe COVID-19 disease, the company said.

BUSINESS
Exclusive: Pfizer says will continue to engage with govt to make its COVID-19 vaccine available in India
In February, the firm had withdrawn an application for emergency-use authorisation of its vaccine in India, after failing to meet the drug regulator’s demand for a local safety and immunogenicity study. Now, as per a new rule, the company is eligible to directly seek EUA from the Drugs Controller General of India.
BUSINESS
Johnson & Johnson submits application to conduct bridge trial of its single-dose COVID-19 vaccine in India
“Johnson & Johnson is partnering with health authorities and the world’s best scientists to provide the safety and efficacy data necessary to support worldwide emergency use of the Janssen COVID-19 vaccine candidate," the company said in a statement to Moneycontrol.

BUSINESS
COVID-19: Raw material crunch pushes vaccine makers to look at indigenization
Bharat Biotech has signed up with a CSIR lab to develop a synthetic process route for adjuvant molecule TLR 7/8 to Covaxin, using locally available chemicals. As export restrictions aren't making life easy, many firms are entering into domestic tie-ups like this.
BUSINESS
Emcure says Gennova is working on backward integration of its mRNA COVID-19 vaccine
The Phase I clinical trial should begin anytime soon. The animal toxicity and safety studies that was conducted as part of preclinical studies have produced desired results, says Vikas Thapar; company looking for foreign tie ups in due course

BUSINESS
Rare clot incidents linked to J&J, AstraZeneca COVID-19 vaccines bring adenovirus vector platform under scanner
Both Sputnik V and J&J used a type of adenovirus called AD26. AstraZeneca-Oxford University's COVID-19 vaccine also uses chimpanzee adenovirus to deliver the antigen.

BUSINESS
Pharma industry sees little impact of Maharashtra's curfew-like restrictions on manufacturing operations
The Maharashtra government has allowed pharma manufacturing units to remain operational with full capacity, as medicines are part of the essential services.

BUSINESS
Government fast tracks approvals for Remdesivir capacity expansion; firms commit to cut price
The current total installed capacity of the seven manufacturers of Remdesivir is 38.80 lakh vials per month.

BUSINESS
Dr Reddy's says it will be importing Sputnik V vaccine from Russia during first quarter of FY22
Dr Reddy's as per its licensing agreement with can produce up to 250 million doses of the Sputnik V vaccine, the deal allows to expand the volumes by mutual consent.

BUSINESS
COVID-19 | 50 million doses of Sputnik V vaccine to be available every month by summer: RDIF
CEO of RDIF Kirill Dmitriev has said the fund is in discussion with companies in India to manufacture the Sputnik V vaccine.

BUSINESS
Explainer: Will govt decision to fast track foreign COVID-19 vaccine approvals ease supply-demand situation?
The government decision means that the local clinical trials or bridging studies that involves testing the vaccine in Indian participants to assess the safety and immunogencity in the local population will now be waived-off.

BUSINESS
Here is how Sputnik V stacks up against Covishield and Covaxin
Over 850 million doses of Sputnik V are going to be produced in India annually, which is sufficient to vaccinate more than 425 million people around the world; 60 countries, including India, have cleared the Russian jab
BUSINESS
India gets 3rd COVID-19 vaccine, DCGI approves Sputnik V for emergency use
Kirill Dmitriev told CNBC-TV18 that they are working out pricing with the government. RDIF has hinted that the Sputnik V will be sold at less than $10 per dose. The vaccine has to be taken in two doses.








