


At a time when countries like India are struggling to scale up testing, researchers at Texas A&M University, Centre for Disease Dynamics, Economics and Policy (CDDEP) and Princeton University have assessed the feasibility of pooled real-time reverse transcription polymerase chain reaction (RT-PCR) testing to vastly increase testing for COVID-19.
The researchers used mathematical analysis to explore efficient pooling strategies using this technique.
For a population containing 256 sampled individuals, where the maximum number of samples in a single pool is 64 (as pooling more samples may be beyond practical testing limits), with only 7.3 tests on the average, it could be possible to distinguish between prevalences of 1 percent and 5 percent with a probability of detection of 95 percent and probability of false alarm of 4 percent.
“This means that rather than testing all 256 individuals in the population, which would be highly costly, with an average of 7.3 tests a 5 percent prevalence of COVID-19 can be detected using this method,” said Krishna Narayanan, professor at Texas A&M University.
The study titled, “Pooling RT-PCR or NGS samples has the potential to cost-effectively generate estimates of COVID-19 prevalence in resource limited environments” by Krishna R Narayanan, Isabel Frost, Anoosheh Heidarzadeh, Katie K Tseng, Sayantan Banerjee, Jacob John and Ramanan Laxminarayan is online at MedRxiv.
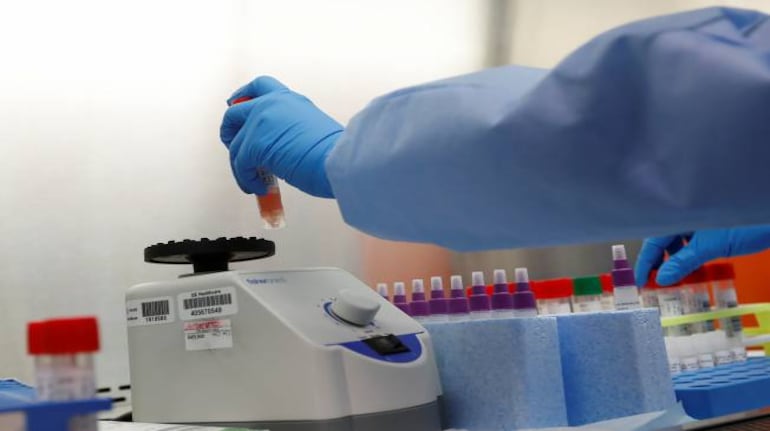
What is pooled testing?A pooled testing method involves putting multiple swab samples in a test tube and testing them using single RT-PCR test.
So, if the test is negative, all the people tested are negative.
If the test is positive, all persons are tested individually. This method can greatly enhance the capacity to test in a low-resources setting, as RT-PCR test is reliable but not scalable.
The number of samples that can be pooled will be determined by the infection rate. More samples can be pooled with less infection. India has only tested little over 50,000 samples. It would have to test more people if it has to slowly ease the lockdown which is in force till April 14.
Testing samples from multiple patients with a single PCR test, also known as pooled sampling, has been used previously in the early stages of the HIV epidemic when PCR costs were high.
"We found that the use of a pooled testing strategy could reduce the time, cost, and resources required whilst identifying infected people in a population and estimating the infection rate. This would allow us to identify community clusters for targeted public health interventions,” says Ramanan Laxminarayan, study author and CDDEP Director and Senior Fellow.
Discover the latest Business News, Sensex, and Nifty updates. Obtain Personal Finance insights, tax queries, and expert opinions on Moneycontrol or download the Moneycontrol App to stay updated!
Find the best of Al News in one place, specially curated for you every weekend.
Stay on top of the latest tech trends and biggest startup news.