


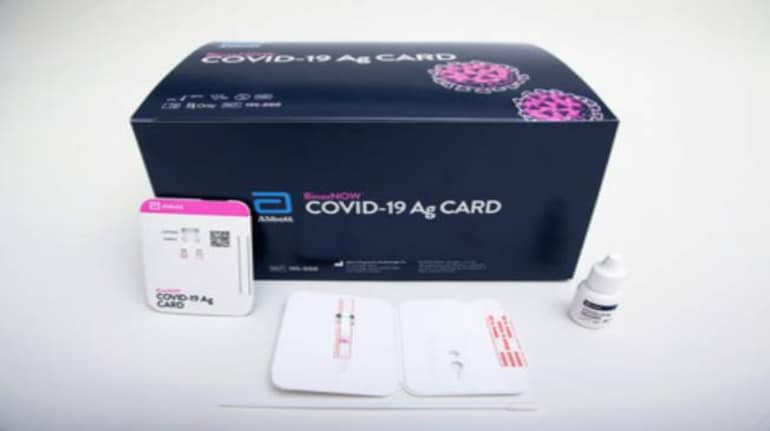
The US Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) for Abbott's 15-minute COVID-19 test. The product, named BinaxNOW, is aimed at eliminating the need for lab tests and thus boosting the nation's capacity to conduct more tests.
The United States is the most-affected country due to the COVID-19 pandemic. It has recorded over 5 million diagnosed cases of COVID-19 and nearly 1,80,000 people have died.
Abbott plans to ship tens of millions of tests in September, ramping to 50 million tests a month at the beginning of October.
BinaxNOW is the size of a credit card and provides results in 15 minutes. Abbott plans to sell this test for $5 (approx Rs 370), which makes it more affordable than a host of other options.
In pics | Here’s the most promising COVID-19 treatments in absence of a vaccine
"BinaxNOW uses a nasal swab to collect specimens from those suspected of having coronavirus and a reactive card to detect COVID-19. No equipment is required to process samples or read test results," Abbott said in a press release on August 27.
Track this LIVE blog for all the latest updates on coronavirus pandemic
Using the product, COVID-19 test can be performed by doctors, nurses, school nurses, medical assistants and technicians, pharmacists, employer occupational health specialists with minimal training and a patient prescription, Abbott claimed.
The company will also launch a mobile app NAVICA for iPhone and Android devices. The app allows people to store, access and display their COVID-19 test results and will work as a temporary digital health pass.
"The status will be renewed each time a person is tested through their healthcare provider together with the date of the test result. Organisations will be able to view and verify the information on a mobile device to facilitate entry into facilities along with hand-washing, social distancing, enhanced cleaning and mask-wearing," it said.
"While BinaxNOW is the hardware that makes knowing your COVID-19 status possible, the NAVICA app is the digital network that allows people to share that information with those who need to know," said Robert B. Ford, president and chief executive officer, Abbott.
"We're taking our know-how from our digitally-connected medical devices and applying it to our diagnostics at a time when people expect their health information to be digital and readily accessible."
Globally, there have been over 2.40 crore confirmed cases of COVID-19. More than 8.20 lakh people have died so far.
Discover the latest Business News, Sensex, and Nifty updates. Obtain Personal Finance insights, tax queries, and expert opinions on Moneycontrol or download the Moneycontrol App to stay updated!
Find the best of Al News in one place, specially curated for you every weekend.
Stay on top of the latest tech trends and biggest startup news.