
BUSINESS
Siemens Healthineers to invest Rs 1,300 crore to set up new campus in Bengaluru
The new campus, to be opened in 2025, would be Siemens Healthineer's largest ever single investment made in India

BUSINESS
COVID-19: Prices of RT-PCR test kits crash over 80%, but not many are happy
Increasing competition and price war see price of one kit dropping from Rs 1,200 in April to Rs 150 now. But quality of kits is the casualty. Early this month, Maharashtra returned 12.5 lakh RT-PCR test kits purchased from a private company, citing faulty results.
BUSINESS
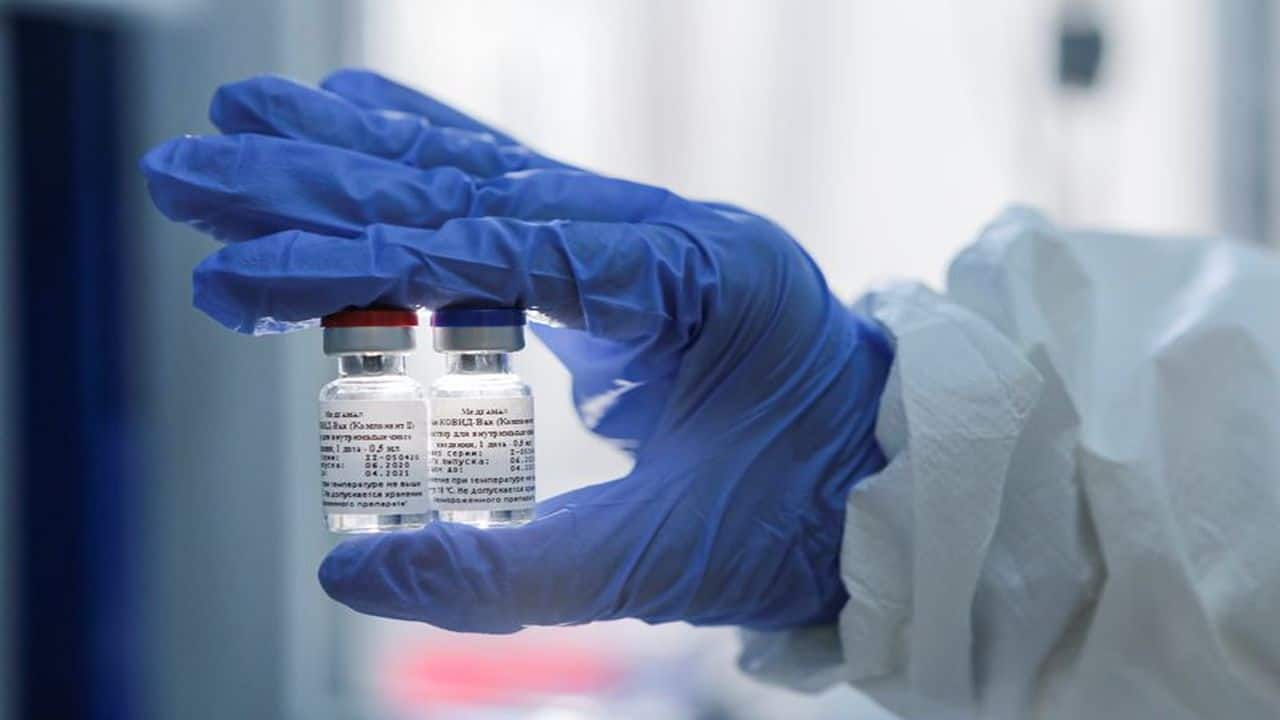
Russia's COVID-19 vaccine Sputnik V makes headway in India
Dr Reddy's gets the nod to conduct human trials for the vaccine while Mankind Pharma signs an agreement with RIDF that will make 300 million doses of Sputnik V available in India.

BUSINESS
Explainer: What does WHO's Solidarity trial data mean for use of Remdesivir against Covid-19
Remdesivir had received regulatory approvals or temporary authorisations to treat COVID-19 in approximately 50 countries including India. The drug has been pre-qualified by WHO.

BUSINESS
WHO Solidarity trial finds Remdesivir has no substantial effect on mortality in COVID-19 patients
The results of the Solidarity trial conducted on 11,266 hospitalised patients in 30 countries found remdesivir, along with three other repurposed drugs hydroxychloroquine, lopinavir/ritonavir and interferon to have little or no effect on 28-day mortality.

BUSINESS
Apollo Hospitals ready to administer 1 million COVID-19 vaccines per day
Apollo Hospitals said it was training 10,000 healthcare workers to be able to administer the COVID-19 vaccine.

BUSINESS
Bharat Biotech fast-tracks Covid-19 vaccine development; company cuts phase-2 size, will seek nod for phase-3
Instead of testing the vaccine candidate on 750 healthy volunteers in Phase-2, as approved for its clinical trial protocol, the company will now be testing it on just 380 volunteers. The 2019 Clinical Trial Rules require all protocol amendments to be submitted to the DCGI in writing along with the Ethics Committee’s letter of approval.

BUSINESS
J&J's pause on late stage clinical trial of COVID-19 vaccine raises concerns
While serious adverse event are not uncommon in clinical trials, they raise concerns, especially when the vaccine development timelines are compressed, leaving less time to sufficiently analyse safety and efficacy of the drug or vaccine

BUSINESS
Covid medicines help Cipla, Glenmark post strong sales growth in a subdued pharma market
The recovery was led by easing of lockdown restrictions and resumption of non-Covid related treatments.

BUSINESS
USFDA inspections taking longer time than usual due to COVID-19 safety protocols
Sun Pharma, Auro Life, Lupin in the queue. Though generally, most inspections are done in a fortnight, now it’s taking almost a month. USFDA is taking precautions to ensure the safety of the investigators and the employees of the facility who assist its staff, say sources.

BUSINESS
Mumbai blackout: Hospitals struggled to keep essential patient services running
As power is restored in most parts of the city, patients and hospital managements heave a sigh of relief.

BUSINESS
Coronavirus vaccine: Bharat Biotech to use ViroVax's adjuvant for Covaxin
Bharat Biotech is currently conducting Phase II human trials of Covaxin after receiving approval from the Drug Controller General of India.

BUSINESS
Pharma wrap: Festivals, winter perfect setting for Covid surge, can't afford to let the guard down
With a vaccine at least three months away, it becomes important for governments to manage the infection rates but it won't be easy.

BUSINESS
Govt to launch behavioural change campaign to check COVID-19 spread
The government feels that there is a laxity in individual behaviour and is worried about upcoming winter and festival seasons, says NITI Aayog Member VK Paul.

BUSINESS
Two years of PMJAY: Treatment worth Rs 15,579 crore provided, but insurers, hospitals seek better rates
Close to 12 million hospitalisation-led treatment has been provided under the Pradhan Mantri Jan Arogya Yojana. However, stakeholders are seeking an upward revision of rates for it to become sustainable for hospitals and insurers.

BUSINESS
COVID-19: Vaccine companies seek buying commitments from govt, global agencies to de-risk investment
Drug makers say it would help them plan better to create capacities. Three potential vaccines candidates from India -- Covishield of Serum Institute with license from AstraZeneca, Covaxin of Bharat Biotech, and ZyCov-D of Zydus Cadila -- have progressed into human trials phase.

BUSINESS
COVID-19: Even as domestic demand for Remdesivir outstrips supply, manufacturers are exporting the drug
One drug company executive says as per the licensing agreement with Gilead, companies will have to cater to 127 countries. Gilead has signed deals with Cipla, Dr. Reddy's, Hetero Labs, Jubilant Life Sciences, Mylan, Syngene and Zydus Cadila.

BUSINESS
Aster puts new hospital projects on hold, renegotiates lease agreements to tide over Covid crisis
Covid has impacted operations and the hospital chain expects normalcy to return only from the fourth quarter. CEO Harish Pillai, said Aster will focus on increasing capacity utilisation at existing projects and build a lab business in regions where it has a strong presence

BUSINESS
Pharma wrap | Coronavirus vaccine: How much will it really cost to immunise India?
Does government have the funds required to foot the COVID-19 vaccination bill?. Read on to know how much it could cost

BUSINESS
Roche Diabetes Care sees traction in glucometer sales as more people opt for self-monitoring
According to Roche Diabetes Care India Country Head Omar Sherief Mohammad, online sales that used to constitute about 5-8 percent of overall sales have increased to 15 percent amid COVID-19.

BUSINESS
Here's a status update on 10 Covid-19 vaccines that are ahead of the pack
India, along with the WHO and the US, has said a vaccine with more than 50 percent efficacy will be acceptable, which means the vaccine must demonstrate it is at least 50 percent more effective than a placebo.

BUSINESS
Coronavirus vaccine update: Novavax begins Phase 3 trials of its COVID-19 vaccine in the UK, to enrol 10,000 participants
Earlier this month Novavax reached an agreement with the Serum Institute of India to produce as many as 2 billion doses a year. The vaccine is expected to be brought online by mid-2021.

BUSINESS
COVID-19: Why nasally administered vaccine experiments are gaining ground among drug makers
Serum Institute’s decision to manufacture Codagenix's intranasal vaccine is the latest example. An intranasal vaccine is simple can even be self-administered. Also, the vaccines being developed by Serum and Bharat Biotech require only a single dose.

BUSINESS
Intranasal COVID-19 vaccine: Bharat Biotech enters licensing pact with Washington University School of Medicine for distribution
While the Phase I trials will take place in St. Louis University’s Vaccine & Treatment Evaluation Unit, Bharat Biotech, upon obtaining the required regulatory approval, will pursue further stages of clinical trials in India and undertake large scale manufacture of the vaccine at its GMP facility located in Genome Valley, Hyderabad.








