



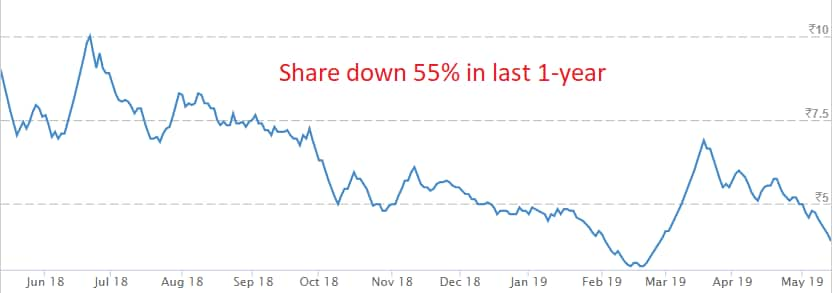
Shares of Orchid Pharma locked at upper circuit on May 15 on the back of USFDA nod for Risedronate Sodium Tablets.
At 09:24 hrs Orchid Pharma was quoting at Rs 4, up Rs 0.17, or 4.44 percent.
There were pending buy orders of 33,257 shares, with no sellers available.
The company has received ANDA approval from United States Food and Drug Administration (USFDA) for Risedronate Sodium Tablets USP, 30 mg and 35 mg.
Risedronate sodium tablets are a bisphosphonate indicated for the treatment and prevention of Osteoporosis.
For more market news, click here
Discover the latest Business News, Sensex, and Nifty updates. Obtain Personal Finance insights, tax queries, and expert opinions on Moneycontrol or download the Moneycontrol App to stay updated!
Find the best of Al News in one place, specially curated for you every weekend.
Stay on top of the latest tech trends and biggest startup news.